化学学报 ›› 2019, Vol. 77 ›› Issue (9): 901-905.DOI: 10.6023/A19050161 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
投稿日期:2019-05-02
发布日期:2019-06-13
通讯作者:
朱钢国
E-mail:gangguo@zjnu.cn
基金资助:
Yang, Junhanga, Fu, Xiaoboa, Lu, Zenghuib, Zhu, Gangguoa*( )
)
Received:2019-05-02
Published:2019-06-13
Contact:
Zhu, Gangguo
E-mail:gangguo@zjnu.cn
Supported by:文章分享
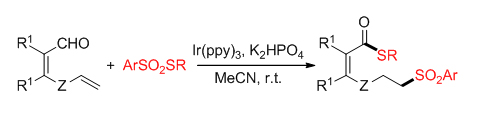
有机硫化合物广泛应用于医药、农药、新材料等领域, 因此, 发展新的碳-硫键形成方法非常重要. 近年来, 烯烃的自由基砜基化反应作为一种温和、有效的有机硫化合物合成策略得到了快速发展, 其中, 烯烃的硫砜基化反应因为能够同时构建两种不同的碳-硫键成了一种非常有吸引力的碳-硫键形成方法. 以硫代磺酸酯同时作砜基化和硫化试剂, 实现了一个可见光催化烯烃砜基化启动的远程醛基碳-氢键直接硫化反应, 一步合成了6-或7-砜基取代的硫酯类化合物. 反应具有优秀的原子经济性, 产率中等到良好, 能兼容各种不同的官能团. 相比传统的烯烃1,2-或1,1-硫砜基化反应, 首次实现了官能团化烯烃的远程硫砜基化反应, 拓展了现有硫砜基化反应方法学. 初步的机理研究表明, 该反应可能经历一个可见光催化的自由基反应历程.
杨俊航, 傅晓波, 卢增辉, 朱钢国. 可见光催化烯烃砜基化启动的远程醛基碳-氢键直接硫化反应[J]. 化学学报, 2019, 77(9): 901-905.
Yang, Junhang, Fu, Xiaobo, Lu, Zenghui, Zhu, Gangguo. Visible-Light Photocatalytic Remote Thiolation of Aldehydes Triggered by Sulfonylation of Alkenes With Thiosulfonates[J]. Acta Chimica Sinica, 2019, 77(9): 901-905.
| Entry | PC | Base | Solvent | Yield/% |
|---|---|---|---|---|
| 1 | Ir(ppy)3 | K2CO3 | MeCN | 60 |
| 2 | Ir(ppy)3 | Na2CO3 | MeCN | 40 |
| 3 | Ir(ppy)3 | Et3N | MeCN | 45 |
| 4 | Ir(ppy)3 | KOAc | MeCN | 46 |
| 5 | Ir(ppy)3 | K3PO4 | MeCN | 44 |
| 6 | Ir(ppy)3 | K2HPO4 | MeCN | 71 |
| 7 | Ru(bpy)3Cl2 | K2HPO4 | MeCN | 30 |
| 8 | Ir[dF(CF3)ppy]2(dtbpy)PF6 | K2HPO4 | MeCN | 54 |
| 9 | Ir(ppy)3 | K2HPO4 | DMF | 23 |
| 10 | Ir(ppy)3 | K2HPO4 | DMSO | 40 |
| 11 | Ir(ppy)3 | K2HPO4 | THF | 40 |
| 12 | Ir(ppy)3 | K2HPO4 | DCM | 68 |
| 13 | Ir(ppy)3 | K2HPO4 | MeCN/H2Ob | 61 |
| 14 | Ir(ppy)3 | K2HPO4 | MeCN c | 79 |
| 15 | Ir(ppy)3d | K2HPO4 | MeCN | 57 |
| 16 | — | K2HPO4 | MeCN | 0 |
| 17 | Ir(ppy)3 | — | MeCN | 50 |
| Entry | PC | Base | Solvent | Yield/% |
|---|---|---|---|---|
| 1 | Ir(ppy)3 | K2CO3 | MeCN | 60 |
| 2 | Ir(ppy)3 | Na2CO3 | MeCN | 40 |
| 3 | Ir(ppy)3 | Et3N | MeCN | 45 |
| 4 | Ir(ppy)3 | KOAc | MeCN | 46 |
| 5 | Ir(ppy)3 | K3PO4 | MeCN | 44 |
| 6 | Ir(ppy)3 | K2HPO4 | MeCN | 71 |
| 7 | Ru(bpy)3Cl2 | K2HPO4 | MeCN | 30 |
| 8 | Ir[dF(CF3)ppy]2(dtbpy)PF6 | K2HPO4 | MeCN | 54 |
| 9 | Ir(ppy)3 | K2HPO4 | DMF | 23 |
| 10 | Ir(ppy)3 | K2HPO4 | DMSO | 40 |
| 11 | Ir(ppy)3 | K2HPO4 | THF | 40 |
| 12 | Ir(ppy)3 | K2HPO4 | DCM | 68 |
| 13 | Ir(ppy)3 | K2HPO4 | MeCN/H2Ob | 61 |
| 14 | Ir(ppy)3 | K2HPO4 | MeCN c | 79 |
| 15 | Ir(ppy)3d | K2HPO4 | MeCN | 57 |
| 16 | — | K2HPO4 | MeCN | 0 |
| 17 | Ir(ppy)3 | — | MeCN | 50 |
| [1] |
Madasu, S. B.; Vekariya, N. A.; Kiran, M. N. V. D. H.; Gupta, B.; Islam, A.; Douglas, P. S.; Babu, K. R . Beilstein J. Org. Chem. 2012, 8, 1400.
doi: 10.3762/bjoc.8.162 |
| [2] |
Fromtling, R. A . Drugs Future 1989, 14, 1165.
doi: 10.1358/dof.1989.014.12.109647 |
| [3] |
Calverley, P. M. A.; Anderson, J. A.; Celli, B.; Ferguson, G. T.; Jenkins, C.; Jones, P. W.; Yates, J. C.; Vestbo, J . N. Engl. J. Med. 2007, 356, 775.
doi: 10.1056/NEJMoa063070 |
| [4] |
(a) Chen, X.; Gan, X.; Chen, J.; Chen, Y.; Wang, Y.; Hu, D.; Song, B . Chin. J. Org. Chem. 2017, 37, 2343.
doi: 10.6023/cjoc201703022 |
|
( 陈学文, 甘秀海, 陈吉祥, 陈永中, 王艳娇, 胡德禹, 宋宝安 . 有机化学, 2017, 37, 2343.)
doi: 10.6023/cjoc201703022 |
|
|
(b) Chen, Y.; Wang, S.; Jiang, Q.; Cheng, C.; Xiao, X.; Zhu, G . J. Org. Chem. 2018, 83, 716.
doi: 10.6023/cjoc201703022 |
|
|
For a review, see:(c) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X . Curr. Top. Med. Chem. 2016, 16, 1200 and references cited therein.
doi: 10.6023/cjoc201703022 |
|
| [5] |
Julia, M.; Paris, J. M . Tetrahedron Lett. 1973, 14, 4833.
doi: 10.1016/S0040-4039(01)87348-2 |
| [6] | Olah, G. A.; Mathew, T.; Prakash, G. K. S . Chem. Commun. 2001,1696. |
| [7] |
(a) Deeming, A. S.; Russell, C. J.; Hennessy, A. J.; Willis, M. C . Org. Lett. 2014, 16, 150.
doi: 10.1021/ol403122a |
|
(b) Wan, Y.; Zhang, J.; Chen, Y.; Kong, L.; Luo, F.; Zhu, G . Org. Biomol. Chem. 2017, 15, 7204.
doi: 10.1021/ol403122a |
|
| [8] |
(a) Zhou, Q.; Gui, J.; Pan, C.-M.; Albone, E.; Cheng, X.; Suh, E. M.; Grasso, L.; Ishihara, Y.; Baran, P. S . J. Am. Chem. Soc. 2013, 135, 12994.
doi: 10.1021/ja407739y |
|
(b) Miao, W.; Zhao, Y.; Ni, C.; Gao, B.; Zhang, W.; Hu, J . J. Am. Chem. Soc. 2018, 140, 880.
doi: 10.1021/ja407739y |
|
|
(c) Griffiths, R. J.; Kong, W. C.; Richards, S. A.; Burley, G. A.; Willis, M. C.; Talbot, E. P. A . Chem. Sci. 2018, 9, 2295.
doi: 10.1021/ja407739y |
|
| [9] |
For selected reviews see: (a) Deeming, A. S.; Emmett, E. J.; Richards-Taylor, C. S.; Willis, M. C. Synthesis. 2014, 46, 2701.
doi: 10.6023/cjoc201702017 |
|
(b) Liu, N.-W.; Liang, S.; Manolikakes, G. Synthesis. 2016, 48, 1939.
doi: 10.6023/cjoc201702017 |
|
|
(c) Qiu, G.; Lai, L.; Cheng, J.; Wu, J . Chem. Commun. 2018, 54, 10405.
doi: 10.6023/cjoc201702017 |
|
|
(d) Guo, W.; Tao, K.; Tan, W.; Zhao, M.; Zheng, L.; Fan, X . Org. Chem. Front.. 2019, 5, DOI: 10.1039/c8qo01353e.
doi: 10.6023/cjoc201702017 |
|
|
(e) Wang, S.; Zheng, Q.; Duan, P.; Liu, W . Chin. J. Org. Chem.. 2017, 37, 1653.
doi: 10.6023/cjoc201702017 |
|
|
( 王守锋, 郑庆飞, 段盼盼, 刘文 , 有机化学, 2017, 37, 1653.)
doi: 10.6023/cjoc201702017 |
|
|
(f) Tan, F.; Xiao, W.; Zeng, G . Chin. J. Org. Chem.. 2017, 37, 824.
doi: 10.6023/cjoc201702017 |
|
|
( 谭芬, 肖文精, 曾国平 , 有机化学, 2017, 37, 824.)
doi: 10.6023/cjoc201702017 |
|
|
(g) Li, S.; Hong, H.; Han, L.; Zhang, T.; Wang, Y.; Zhu, N . Chin. J. Org. Chem.. 2018, 38, 304.
doi: 10.6023/cjoc201702017 |
|
|
( 李闪闪, 洪海龙, 韩利民, 张田苗, 王云龙, 竺宁, , 有机化学, 2018, 38, 304.)
doi: 10.6023/cjoc201702017 |
|
|
(h) Li, S.-S.; Wang, J . Acta Chim. Sinica . 2018, 76, 913.
doi: 10.6023/cjoc201702017 |
|
|
( 李树森, 王剑波 , 化学学报, 2018, 76, 913.)
doi: 10.6023/cjoc201702017 |
|
|
For a report, see:(i) Yuan, Y.; Cao, Y.; Qiao, J.; Lin, Y.; Jiang, X.; Weng, Y.; Tang, S.; Lei, A . Chin. J. Chem. 2019, 37, 49.
doi: 10.6023/cjoc201702017 |
|
| [10] |
(a) Liu, T.; Li, Y.; Lai, L.; Cheng, J.; Sun, J.; Wu, J. Org. Lett. 2018, 20, 3605.
doi: 10.1021/acs.orglett.8b01385 |
|
(b) Ye, S.; Zheng, D.; Wu, J.; Qiu, G. Chem. Commun. 2019, 55, 2214.
doi: 10.1021/acs.orglett.8b01385 |
|
| [11] |
(a) Meyer, A. U.; Jäger, S.; Hari, D. P.; König, B . Adv. Synth. Catal. 2015, 357, 2050.
doi: 10.1002/adsc.v357.9 |
|
(b) Zhang, G.; Zhang, L.; Yi, H. Luo, Y.; Qi, X.; Tung, C.-H.; Wu, L.-Z.; Lei, A . Chem. Commun. 2016, 52, 10407.
doi: 10.1002/adsc.v357.9 |
|
|
(c) Ratushnyy, M.; Kamenova, M.; Gevorgyan, V. Chem. Sci. 2018, 9, 7193.
doi: 10.1002/adsc.v357.9 |
|
|
(d) Sun, D.; Zhang, R . Org. Chem. Front. 2018, 5, 92.
doi: 10.1002/adsc.v357.9 |
|
|
(e) Cai, S.; Xu, Y.; Chen, D.; Li, L.; Chen, Q.; Huang, M.; Weng, W . Org. Lett. 2016, 128, 7440.
doi: 10.1002/adsc.v357.9 |
|
| [12] |
(a) Quebatte, L.; Thommes, K.; Severin, K . J. Am. Chem. Soc. 2006, 128, 7440.
doi: 10.1021/ja0617542 |
|
(b) Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O . ACS Catal. 2019, 9, 1103.
doi: 10.1021/ja0617542 |
|
|
(c) Taniguchi, T.; Idota, A.; Ishibashi, H . Org. Biomol. Chem. 2011, 9, 3151.
doi: 10.1021/ja0617542 |
|
|
(d) Pagire, S. K.; Paria, S.; Reiser, O . Org. Lett. 2016, 18, 2016
doi: 10.1021/ja0617542 |
|
|
(e) Xiong, Y.; Sun, Y.; Zhang, G . Org. Lett. 2018, 20, 6250.
doi: 10.1021/ja0617542 |
|
|
(f) Rao, W.-H.; Jiang, L.-L.; Liu, X.-M.; Chen, M.-J.; Chen, F.-Y.; Jiang, X.; Zhao, J.-X.; Zou, G.-D.; Zhou, Y.-Q.; Tang, L . Org. Lett. 2019, 21, 2890.
doi: 10.1021/ja0617542 |
|
|
(g) Wang, H.; Wang, G.; Lu, Q.; Chiang, C.-W.; Peng, P.; Zhou, J.; Lei, A . Chem. Eur. J. 2016, 22, 14489.
doi: 10.1021/ja0617542 |
|
|
(h) Yuan, Y.; Cao, Y.; Lin, Y.; Li, Y.; Huang, Z.; Lei, A . ACS Catal. 2018, 8, 10871.
doi: 10.1021/ja0617542 |
|
| [13] |
(a) Gao, Y.; Mei, H.; Han, J.; Pan, Y . Chem. Eur. J. 2018, 24, 17205.
doi: 10.1002/chem.201804157 |
|
(b) Sun, J.; Li, P.; Guo, L.; Yu, F.; He, Y.-P.; Chu, L. Chem. Commun. 2018, 54, 3162.
doi: 10.1002/chem.201804157 |
|
|
(c) Pirenne, V.; Kurtay, G.; Voci, S.; Bouffier, L.; Sojic, N.; Robert, F.; Bassani, D. M.; Landais, Y . Org. Lett. 2018, 20, 4521.
doi: 10.1002/chem.201804157 |
|
| [14] |
(a) Chen, Z.-Z.; Liu, S.; Hao, W.-J.; Xu, G.; Wu, S.; Miao, J.-N.; Jiang, B.; Wang, S.-L.; Tu, S.-J.; Li, G . Chem. Sci. 2015, 6, 6654.
doi: 10.1039/C5SC02343B |
|
(b) Huang, M.-H.; Zhu, C.-F.; He, C.-L.; Zhu, Y.-L.; Hao, W.-J.; Wang, D.-C.; Tu, S.-J.; Jiang, B . Org. Chem. Front. 2018, 5, 1643.
doi: 10.1039/C5SC02343B |
|
|
(c) Wu, W.; Yi, S.; Yu, Y.; Huang, W.; Jiang, H . J. Org. Chem. 2017, 82, 1224.
doi: 10.1039/C5SC02343B |
|
|
(d) Cao, X.; Cheng, X.; Xuan, J . Org. Lett. 2018, 20, 449.
doi: 10.1039/C5SC02343B |
|
| [15] |
(a) Zhu, D.; Shao, X.; Hong, X.; Lu, L.; Shen, Q . Angew. Chem., Int. Ed. 2016, 55, 15807.
doi: 10.1002/anie.201609468 |
|
(b) Zhao, Q.; Lu, L.; Shen, Q . Angew. Chem., Int. Ed. 2017, 56, 11575.
doi: 10.1002/anie.201609468 |
|
| [16] |
(a) Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Chem. Sci. 2017, 8, 2610.
doi: 10.1039/C6SC05093J |
|
(b) Huang, S.; Thirupathi, N.; Tung, C.-H.; Xu, Z . J. Org. Chem. 2018, 83, 9449.
doi: 10.1039/C6SC05093J |
|
| [17] |
He, F.-S.; Wu, Y.; Zhang, J.; Xia, H.; Wu, J . Org. Chem. Front. 2018, 5, 2940.
doi: 10.1039/C8QO00824H |
| [18] |
(a) Cheng, C.; Liu, S.; Lu, D.; Zhu, G. Org. Lett. 2016, 18, 2852.
doi: 10.1021/acs.orglett.6b01113 |
|
(b) Nie, X.; Cheng, C.; Zhu, G . Angew. Chem., Int. Ed. 2017, 56, 1898.
doi: 10.1021/acs.orglett.6b01113 |
|
|
(c) Jin, W.; Zhou, Y.; Zhao, Y.; Ma, Q.; Kong, L.; Zhu, G . Org. Lett. 2018, 20, 1435.
doi: 10.1021/acs.orglett.6b01113 |
|
|
(d) Wan, Y.; Shang, T.; Lu, Z. Zhu, G . Org. Lett. 2019, 21, 4187.
doi: 10.1021/acs.orglett.6b01113 |
|
| [19] |
For selected reviews on photocatalysis, see:(a) Narayanam, J. M. R.; Stephenson, C. R. J . Chem. Soc. Rev. 2011, 40, 102.
doi: 10.1039/B913880N |
|
(b) Xuan, J.; Xiao, W.-J . Angew. Chem., Int. Ed. 2012, 51, 6828.
doi: 10.1039/B913880N |
|
|
(c) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C . Chem. Rev. 2013, 113, 5322.
doi: 10.1039/B913880N |
|
|
(d) Xi, Y.; Yi, H.; Lei, A . Org. Biomol. Chem. 2013, 11, 2387.
doi: 10.1039/B913880N |
|
|
(e) Yu, S.; Zhang, Y.; Wang, R.; Jiang, H.; Cheng, Y.; Kadi, A.; Fun, H.-K . Synthesis. 2014, 2711.
doi: 10.1039/B913880N |
|
|
(f) Xie, J.; Jin, H.; Xu, P.; Zhu, C . Tetrahedron Lett. 2014, 55, 36.
doi: 10.1039/B913880N |
|
|
(g) Wang, C.; Lu, Z . Org. Chem. Front. 2015, 2, 179.
doi: 10.1039/B913880N |
|
|
(h) Matsui, J. K.; Lang, S. B.; Heitz, D. R.; Molander, G. A . ACS Catal. 2017, 7, 2563.
doi: 10.1039/B913880N |
|
| [20] |
For selected reviews see: (a) Hu, X.-Q.; Chen, J.-R.; Xiao, W.-J . Angew. Chem., Int. Ed. 2017, 56, 1960.
doi: 10.1002/anie.201611463 |
|
(b) Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C . Chem. Soc. Rev. 2018, 47, 654.
doi: 10.1002/anie.201611463 |
|
|
(c) Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A . Synjournal 2018, 50, 1569.
doi: 10.1002/anie.201611463 |
|
|
(d) Nechab, M.; Mondal, S.; Bertrand, M. P . Chem. Eur. J.. 2018, 20, 16034.
doi: 10.1002/anie.201611463 |
|
|
For selected reports involving 1,n-HAT since 2018, see:(e) Short, M. A.; Blackburn, J. M.; Roizen, J. L . Angew. Chem., Int. Ed. 2018, 57, 296.
doi: 10.1002/anie.201611463 |
|
|
(f) Dauncey, E. M.; Morcillo, S. P.; Douglas, J. J.; Sheikh, N. S.; Leonori, D . Angew. Chem., Int. Ed. 2018, 57, 744.
doi: 10.1002/anie.201611463 |
|
|
(g) Wu, X.; Wang, M.; Huan, L.; Wang, D.; Wang, J.; Zhu, C . Angew. Chem., Int. Ed. 2018, 57, 1640.
doi: 10.1002/anie.201611463 |
|
|
(h) Wu, S.; Wu, X.; Wang, D.; Zhu, C . Angew. Chem., Int. Ed. 2019, 58, 1499.
doi: 10.1002/anie.201611463 |
|
|
(i) Jiang, H.; Studer, A . Angew. Chem., Int. Ed. 2018, 57, 1692.
doi: 10.1002/anie.201611463 |
|
|
(j) Xia, Y.; Wang, L.; Studer, A . Angew. Chem., Int. Ed. 2018, 57, 12940.
doi: 10.1002/anie.201611463 |
|
|
(k) Ratushnyy, M.; Parasram, M.; Wang, Y.; Gevorgyan, V . Angew. Chem., Int. Ed. 2018, 57, 2712.
doi: 10.1002/anie.201611463 |
|
|
(l) Chuentragool, P.; Yadagiri, D.; Morita, T.; Sarkar, S.; Parasram, M.; Wang, Y.; Gevorgyan, V . Angew. Chem., Int. Ed. 2019, 58, 1794.
doi: 10.1002/anie.201611463 |
|
|
(m) Na, C. G.; Alexanian, E. J . Angew. Chem., Int. Ed. 2018, 57, 13106.
doi: 10.1002/anie.201611463 |
|
|
(n) Li, Z.; Wang, Q.; Zhu, J . Angew. Chem., Int. Ed. 2018, 57, 13288.
doi: 10.1002/anie.201611463 |
|
|
(o) Bao, X.; Wang, Q.; Zhu, J . Angew. Chem., Int. Ed. 2019, 58, 2139.
doi: 10.1002/anie.201611463 |
|
|
(p) Kim, I.; Park, B.; Kang, G.; Kim, J.; Jung, H.; Lee, H.; Baik, M.-H.; Hong, S . Angew. Chem., Int. Ed. 2018, 57, 15517.
doi: 10.1002/anie.201611463 |
|
|
(q) Guan, H.; Sun, S.; Mao, Y.; Chen, L.; Lu, R.; Huang, J.; Liu, L . Angew. Chem., Int. Ed. 2018, 57, 11413.
doi: 10.1002/anie.201611463 |
|
|
(r) Hu, A.; Guo, J.-J.; Pan, H.; Tang, H.; Gao, Z.; Zuo, Z . J. Am. Chem. Soc. 2018, 140, 1612.
doi: 10.1002/anie.201611463 |
|
|
(s) An, X.-D.; Jiao, Y.-Y.; Zhang, H.; Gao, Y.; Yu, S . Org. Lett. 2018, 20, 401.
doi: 10.1002/anie.201611463 |
|
|
(t) Zhu, Y.; Huang, K.; Pan, J.; Qiu, X.; Luo, X.; Qin, Q.; Wei, J.; Wen, X.; Zhang, L.; Jiao, N . Nat. Commun. 2018, 9, 2625.
doi: 10.1002/anie.201611463 |
|
|
(u) Li, G.-X.; Hu, X.; He, G.; Chen, G . Chem. Sci. 2019, 10, 688.
doi: 10.1002/anie.201611463 |
|
|
(v) Zhang, Z.; Stateman, L. M.; Nagib, D. A . Chem. Sci. 2019, 10, 1207.
doi: 10.1002/anie.201611463 |
|
|
(w) Wu, K.; Wang, L.; Colón-Rodríguez, S.; Flechsig, G.-U.; Wang, T . Angew. Chem., Int. Ed. 2019, 58, 1774.
doi: 10.1002/anie.201611463 |
|
|
(x) Liu, Z.; Xiao, H.; Zhang, B.; Shen, H.; Zhu, L.; Li, C . Angew. Chem., Int. Ed. 2019, 58, 2510.
doi: 10.1002/anie.201611463 |
| [1] | 刘嘉文, 林玮璜, 王惟嘉, 郭学益, 杨英. Cu1.94S-SnS纳米异质结的合成及其光催化降解研究[J]. 化学学报, 2023, 81(7): 725-734. |
| [2] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [3] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [4] | 张冠华, 杨子涵, 马越. 混合工艺对氧化物/硫化物复合固态电解质电化学性能的影响[J]. 化学学报, 2023, 81(10): 1387-1393. |
| [5] | 李奎琛, 郑开元, 何静嘉, 金泽浩, 何秋, 王丽丽. 单相硫化锌量子点制作白光发光二极管(WQLEDs)[J]. 化学学报, 2023, 81(10): 1327-1333. |
| [6] | 梁世硕, 康树森, 杨东, 胡建华. 锂金属负极界面修饰及其在硫化物全固态电池中的应用[J]. 化学学报, 2022, 80(9): 1264-1268. |
| [7] | 舒恒, 包义德日根, 那永. CdS基纳米管光催化氧化5-羟甲基糠醛选择性生成2,5-呋喃二甲醛[J]. 化学学报, 2022, 80(5): 607-613. |
| [8] | 杨思明, 刘爱荣, 刘静, 刘钊丽, 张伟贤. 硫化纳米零价铁研究进展: 合成、性质及环境应用[J]. 化学学报, 2022, 80(11): 1536-1554. |
| [9] | 杨民, 叶柏柏, 陈健强, 吴劼. 可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应[J]. 化学学报, 2022, 80(1): 11-15. |
| [10] | 马智烨, 叶丽, 吴雨桓, 赵彤. B,N-SnO2/TiO2光催化剂的制备及其光催化性能研究[J]. 化学学报, 2021, 79(9): 1173-1179. |
| [11] | 徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟. 退役锂离子电池碳/硫协同选择性提锂技术[J]. 化学学报, 2021, 79(8): 1073-1081. |
| [12] | 陈锋, 程晓琴, 赵振新, 王晓敏. 分级多孔N, P共掺杂rGO改性隔膜增强锂硫电池的循环稳定性[J]. 化学学报, 2021, 79(7): 941-947. |
| [13] | 白子昂, 陈瑞兴, 庞宏伟, 王祥学, 宋刚, 于淑君. 硫化纳米零价铁对水中U(VI)的高效去除研究[J]. 化学学报, 2021, 79(10): 1265-1272. |
| [14] | 董奎, 刘强, 吴骊珠. 放氢交叉偶联反应[J]. 化学学报, 2020, 78(4): 299-310. |
| [15] | 张振, 龚莉, 周晓渝, 颜思顺, 李静, 余达刚. 二氧化碳参与的自由基型烯烃双官能团化反应[J]. 化学学报, 2019, 77(9): 783-793. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||