化学学报 ›› 2019, Vol. 77 ›› Issue (9): 841-849.DOI: 10.6023/A19050183 上一篇 下一篇
所属专题: 有机自由基化学
综述
投稿日期:2019-05-15
发布日期:2019-08-14
通讯作者:
肖丽,王挺
E-mail:xiao.221@buckeyemail.osu.edu;twang3@albany.edu
作者简介:肖丽, 2008~2012年就读于武汉大学化学与分子科学学院; 2012~2017年就读于俄亥俄州立大学化学与生物化学学院有机化学专业(2017年获得博士学位, 导师: Craig Forsyth教授); 2018年至今在美国Biogen生物制药公司从事神经领域药物合成与工艺相关研究工作.|李嘉恒, 1988年出生于吉林省吉林市. 2012年毕业于吉林大学化学学院后在东北师范大学(NENU)王芒教授指导下开展有机氟化学研究, 并于2017年获得理学博士学位. 毕业后就职于吉林大学第一医院表观遗传医药研究所从事新药研发. 2018年8月加入纽约州立大学-奥尔巴尼分校(SUNY-Albany)王挺课题组进行博士后研究, 目前主要围绕新型有机光催化剂的合成及其应用开展相关研究工作.|王挺, 2001~2005年就读于天津大学药物科学与技术学院, 2011年在俄亥俄州立大学获博士学位. 2011至2015年在斯隆-凯特琳癌症研究中心从事博士后工作. 2015年9月加入纽约州立大学-奥尔巴尼分校(SUNY-Albany)化学系. 目前主要研究领域: 有机光催化合成方法学的研究, 以及运用新的光催化方法构建有生物活性的小分子天然产物, 糖类化合物, 多肽和糖肽化合物.
Xiao, Lia*( ), Li, Jiahengb, Wang, Tingb*(
), Li, Jiahengb, Wang, Tingb*( )
)
Received:2019-05-15
Published:2019-08-14
Contact:
Xiao, Li,Wang, Ting
E-mail:xiao.221@buckeyemail.osu.edu;twang3@albany.edu
文章分享
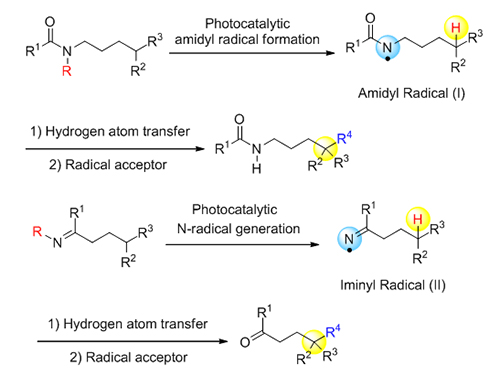
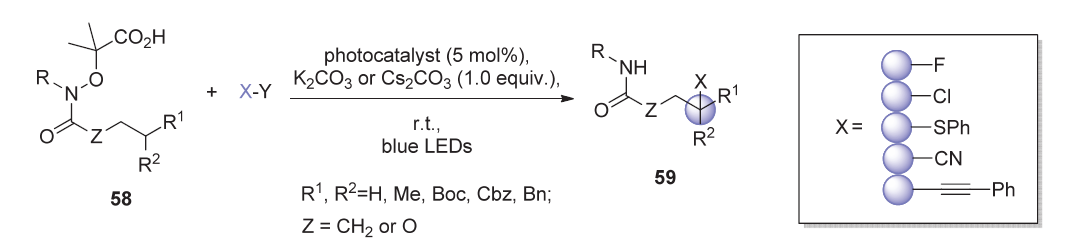
远程sp 3碳氢键官能团化反应近年来引起广泛关注, 可见光催化的氮自由基导向选择性官能化这一技术的出现使得该领域的发展取得了可喜的进展. 该策略以氮自由基介导的Hoffman-L?ffler-Freytag (HLF)反应为基础, 通过在可见光照射下激发态自由基的生成和1,5-攫取氢原子(1,5-HAT)过程, 利用反应过程中形成的自由基中间体, 实现对远程sp 3碳-氢键的修饰. 本综述就可见光催化的氮自由基导向远程碳氢官能团化反应近年来取得的进展进行简要总结.
肖丽, 李嘉恒, 王挺. 可见光催化的氮自由基导向远程sp 3碳氢官能团化反应[J]. 化学学报, 2019, 77(9): 841-849.
Xiao, Li, Li, Jiaheng, Wang, Ting. Visible-Light-Induced N-Radical Directed Remote Functionalization of sp 3 C-H Bonds[J]. Acta Chimica Sinica, 2019, 77(9): 841-849.
| [1] |
Prier C. K.; Rankic D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
doi: 10.1021/cr300503r |
| [2] |
Hoplinson M. N.; Sahoo B.; Li J.-L.; Glorius F . Chem. -Eur. J. 2014, 20, 3874.
doi: 10.1002/chem.201304823 |
| [3] |
Kärkäs M. D.; Porco J. A. Jr.; Stephenson, C. R. J. Chem. Rev. 2016, 116, 9683.
doi: 10.1021/acs.chemrev.5b00760 |
| [4] |
Xuan J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828.
doi: 10.1002/anie.201200223 |
| [5] |
Narayanam J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102.
doi: 10.1039/B913880N |
| [6] |
Yoon, T. P. ACS Catal. 2013, 3, 895.
doi: 10.1021/cs400088e |
| [7] |
Nicewicz D. A.; Nguyen T. M . ACS Catal. 2014, 4. 355.
doi: 10.1021/cs400956a |
| [8] |
Fukuzumi S.; Ohkubo, K. Org. Biomol. Chem. 2014, 12, 6059.
doi: 10.1039/C4OB00843J |
| [9] |
Hari D. P.; König B . Chem. Commun. 2014, 50, 6688.
doi: 10.1039/C4CC00751D |
| [10] |
Martin M. L.; Santos-Juanes, L.; Arques, A.; Amat, A. M.; Miranda, M. A . Chem. Rev. 2012, 112, 1710.
doi: 10.1021/cr2000543 |
| [11] |
Nicewicz D. A.; Romero, N. A. Chem. Rev. 2016, 116, 10075.
doi: 10.1021/acs.chemrev.6b00057 |
| [12] | Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J . Chem. Soc. Rev. 2016, 45, 2044. |
| [13] |
Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan, D. W. C. Nat. Rev. Chem. 2017, 1, 52.
doi: 10.1038/s41570-017-0052 |
| [14] |
Tellis J. C.; Kelly C. B.; Primer D. N.; Jouffroy M.; Patel N. R.; Molander, G. A. Acc. Chem. Res. 2016, 49, 1429.
doi: 10.1021/acs.accounts.6b00214 |
| [15] |
Chen Y.-Y.; Lu L.-Q.; Yu D.-G.; Zhu C.-J.; Xiao, W.-J. Sci. China Chem. 2019, 62, 24.
doi: 10.1007/s11426-018-9399-2 |
| [16] |
He J.; Wasa M.; Chan K. S.; Shao Q.; Yu J.-Q . Chem. Rev. 2016, 117, 8754.
doi: 10.1021/acs.chemrev.6b00622 |
| [17] | Gutekunst W. R.; Baran P. S . Chem. Soc. Rev. 2011, 40, 1976. |
| [18] |
Yamaguchi J.; Yamaguchi A. D.; Itami K . Angew. Chem., Int. Ed. 2012, 51, 8960.
doi: 10.1002/anie.201201666 |
| [19] |
Huang Z.; Lim H. N.; Mo F.; Young M. C.; Dong G . Chem. Soc. Rev. 2015, 44, 7764.
doi: 10.1039/C5CS00272A |
| [20] |
(a) Feng J.; Li B.; Jiang J.; Zhang M.; Ouyang W.; Li C.; Fu Y.; Gu, Z. Chin. J. Chem. 2018, 36, 11.
doi: 10.1002/cjoc.201700618 |
|
(b) Pei P.; Zhang F.; Yi H.; Lei, A. Acta Chim. Sinica 2017, 75, 15.
doi: 10.1002/cjoc.201700618 |
|
|
(裴朋昆, 张凡, 易红, 雷爱文, 化学学报, 2017, 75, 15.)
doi: 10.1002/cjoc.201700618 |
|
|
(c) Zhong, J.; Meng, Q.; Chen, B.; Tung, C.-H.; Wu, L.-Z . Acta Chim. Sinica. 2017, 75, 34.
doi: 10.1002/cjoc.201700618 |
|
|
(钟建基, 孟庆元, 陈彬, 佟振合, 吴骊珠, 化学学报, 2017, 75, 34.)
doi: 10.1002/cjoc.201700618 |
|
| [21] |
Chiba S.; Chen H . Org. Biomol. Chem. 2014, 12, 4051.
doi: 10.1039/C4OB00469H |
| [22] |
Robertson J.; Pillai J.; Lush, R. K. Chem. Soc. Rev. 2001, 30, 94.
doi: 10.1039/b000705f |
| [23] |
Mayer J. M . Acc. Chem. Res. 2010, 44, 36.
doi: 10.1021/ar100093z |
| [24] |
Protti S.; Fagnoni M.; Ravelli D . ChemCatChem 2015, 7, 1516.
doi: 10.1002/cctc.v7.10 |
| [25] |
Wolff M. E . Chem. Rev. 1963, 63, 55.
doi: 10.1021/cr60221a004 |
| [26] |
Qin Q.; Yu S . Org. Lett. 2015, 17, 1894.
doi: 10.1021/acs.orglett.5b00582 |
| [27] |
Choi G. J.; Zhu Q.; Miller D. C.; Gu C. J.; Knowles R. R . Nature 2016, 539, 268.
doi: 10.1038/nature19811 |
| [28] |
Chu J. C. K.; Rovis T . Nature 2016, 539, 272.
doi: 10.1038/nature19810 |
| [29] |
Chen D.-F.; Chu J. C. K.; Rovis, T. J. Am. Chem. Soc. 2017, 139, 14897.
doi: 10.1021/jacs.7b09306 |
| [30] |
Yuan W.; Zhou Z.; Gong L.; Meggers E . Chem. Commun. 2017, 53, 8964.
doi: 10.1039/C7CC04941B |
| [31] |
Shu W.; Nevado, C. Angew. Chem., Int. Ed. 2017, 56, 1881.
doi: 10.1002/anie.201609885 |
| [32] |
Chen H.; Guo L.; Yu S . Org. Lett. 2018, 20, 6255.
doi: 10.1021/acs.orglett.8b02737 |
| [33] |
Shen X.; Zhao J.; Yu S . Org. Lett. 2018, 20, 5523.
doi: 10.1021/acs.orglett.8b02540 |
| [34] |
Xia Y.; Wang L.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 12940.
doi: 10.1002/anie.201807455 |
| [35] |
Dauncey E. M.; Morcillo S. P.; Douglas J. J.; Sheikh N. S.; Leonori, D. Angew. Chem., Int. Ed. 2018, 57, 744.
doi: 10.1002/anie.201710790 |
| [36] |
Jiang H.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 1692.
doi: 10.1002/anie.v57.6 |
| [37] |
Morcillo S. P.; Dauncey E. M.; Kim J. H.; Douglas J. J.; Sheikh N. S.; Leonori, D. Angew. Chem., Int. Ed. 2018, 57, 12945.
doi: 10.1002/anie.201807941 |
| [38] |
Wappes E. A.; Vanitcha A.; Nagib, D. A. Chem. Sci. 2018, 9, 4500.
doi: 10.1039/C8SC01214H |
| [39] |
Wu K.; Wang L.; Colόn-Rodrίguez S.; Flechsig G.; Wang, T. Angew. Chem., Int. Ed. 2019, 58, 1774.
doi: 10.1002/anie.201811004 |
| [40] | Xu B.; Tambar, U. K. ACS Catal. 2019, 9, 4727. |
| [1] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [2] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [3] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [4] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [5] | 李珊, 路俊欣, 刘杰, 蒋绿齐, 易文斌. 氟烷基亚磺酸钠盐电化学合成α-氟烷基酮[J]. 化学学报, 2024, 82(2): 110-114. |
| [6] | 杜思南, 赵丽曼, 张泽新, 陈国颂. 甘露糖修饰的微马达的制备及其免疫功能初探★[J]. 化学学报, 2023, 81(7): 741-748. |
| [7] | 任妍妍, 李欣, 韩英锋. 基于氮杂环卡宾蓝光有机自由基的合成及其光学性质研究★[J]. 化学学报, 2023, 81(7): 735-740. |
| [8] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [9] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [10] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [11] | 杨洁, 凌琳, 李玉学, 吕龙. 高氯酸铵热分解机理的密度泛函理论研究[J]. 化学学报, 2023, 81(4): 328-337. |
| [12] | 赵亚婷, 刘帆, 汪秋安, 夏吾炯. 可见光促进(氮杂)芳香胺与重氮乙酸乙酯的N-烷基化反应[J]. 化学学报, 2023, 81(2): 111-115. |
| [13] | 陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623. |
| [14] | 张红丹, 兰欣雨, 程鹏. 羟基自由基辅助沸石分子筛合成的研究进展[J]. 化学学报, 2023, 81(1): 100-110. |
| [15] | 解众舒, 薛中鑫, 许子文, 李倩, 王洪宇, 李维实. 石墨相氮化碳的共轭交联修饰及其对可见光催化产氢性能的影响[J]. 化学学报, 2022, 80(9): 1231-1237. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||