化学学报 ›› 2021, Vol. 79 ›› Issue (10): 1232-1243.DOI: 10.6023/A21060260 上一篇 下一篇
综述
投稿日期:2021-06-09
发布日期:2021-07-20
通讯作者:
杨勇
作者简介: |
谢佶晟, 2021年于厦门大学化学系获学士学位, 研究方向为钠离子电池层状过渡金属氧化物正极材料的失效及改性机理研究. |
 |
肖竹梅, 2018年于南开大学化学系获学士学位, 现为厦门大学化学化工学院杨勇教授课题组硕士研究生. 研究方向为锂、钠离子电池中高比能正极材料的反应机理研究. |
 |
左文华, 2020年于厦门大学获得博士学位, 师从杨勇教授, 目前主要从事钠离子电池层状过渡金属氧化物正极材料研究. |
 |
杨勇, 厦门大学闽江计划特聘教授, 博士生导师, 国家杰出青年科学基金获得者. 1992年获厦门大学理学博士学位, 1997~1998年任英国牛津大学访问科学家. 主要研究方向为能源电化学、材料物理化学及表面物理化学. |
基金资助:
Jisheng Xiea, Zhumei Xiaoa, Wenhua Zuoa, Yong Yanga,b( )
)
Received:2021-06-09
Published:2021-07-20
Contact:
Yong Yang
Supported by:文章分享

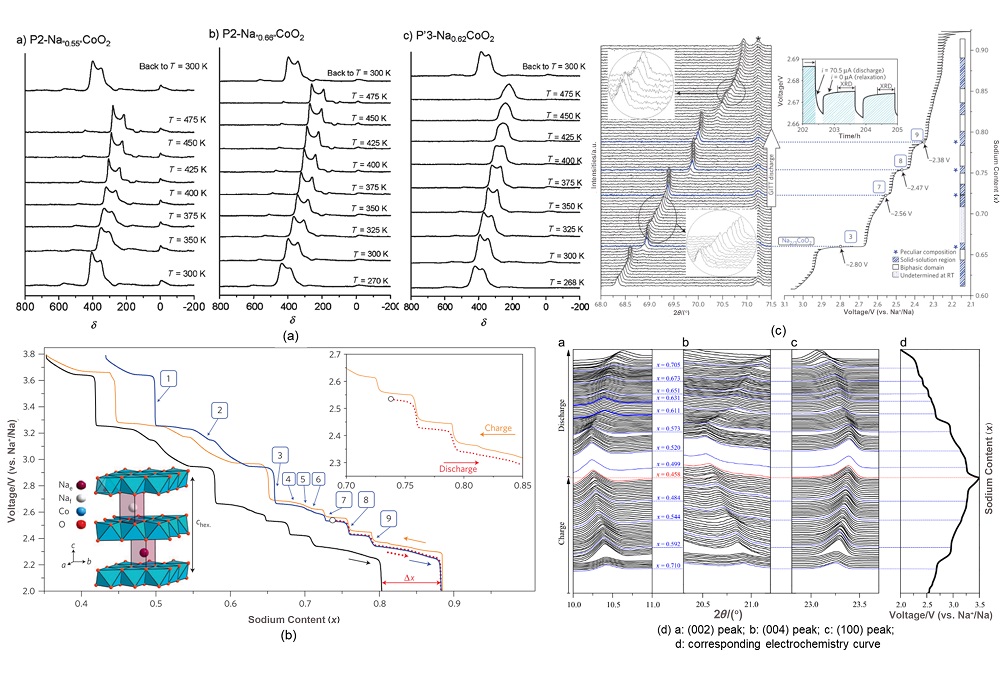
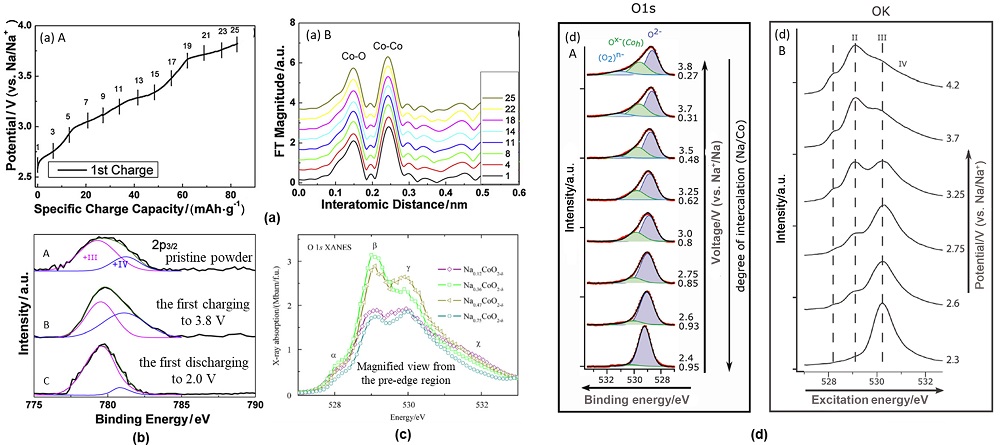
钠离子电池凭借分布广泛和低成本的钠资源在大规模电化学能量存储领域受到广泛关注. 层状过渡金属氧化物作为一种重要的钠离子电池正极材料, 具有比容量高、电化学可逆性相对较好和化学组成丰富且可调的特征, 得到广泛关注. 其中钴酸钠是一种典型层状过渡金属氧化物, 自20世纪80年代就得到大量研究. 由于钴酸钠含有丰富的电化学信息, 基于其充放电过程进行的机理研究对理解钠离子电池层状氧化物体系具有重要意义. 因此在介绍钴酸钠的常见结构类型与合成相图的基础上, 本文着重综述了不同结构钴酸钠在充放电过程中结构变化和电荷补偿机理的研究进展, 同时讨论了上述机制对电化学性能的影响. 本综述旨在为深入研究并建立层状过渡金属氧化物正极材料电化学过程中的构效关系提供支持.
谢佶晟, 肖竹梅, 左文华, 杨勇. 钠离子电池钴酸钠正极材料研究进展[J]. 化学学报, 2021, 79(10): 1232-1243.
Jisheng Xie, Zhumei Xiao, Wenhua Zuo, Yong Yang. Research Progresses of Sodium Cobalt Oxide as Cathode in Sodium Ion Batteries[J]. Acta Chimica Sinica, 2021, 79(10): 1232-1243.
| 名称a | 优点 | 缺点 |
|---|---|---|
| NaMnO2 | Mn自然丰度高, 理论容量高(243 mAh/g) | 存在Jahn-Teller畸变, 充放电阶段多阶梯状曲线, 结构稳定性差, 循环性能差 |
| NaFeO2 | Fe自然丰度极高, 低电压下充放电平台稳定, 电化学可逆性好 | 质量比容量低, 高电位下存在不可逆相变, 循环稳定性差 |
| NaCoO2 | 低电压下电化学可逆性较好, 离子电导率高 | Co自然丰度极低, 成本高, 容量低, 充放电曲线多平台, 倍率性能差, 高电位下循环性能差 |
| 名称a | 优点 | 缺点 |
|---|---|---|
| NaMnO2 | Mn自然丰度高, 理论容量高(243 mAh/g) | 存在Jahn-Teller畸变, 充放电阶段多阶梯状曲线, 结构稳定性差, 循环性能差 |
| NaFeO2 | Fe自然丰度极高, 低电压下充放电平台稳定, 电化学可逆性好 | 质量比容量低, 高电位下存在不可逆相变, 循环稳定性差 |
| NaCoO2 | 低电压下电化学可逆性较好, 离子电导率高 | Co自然丰度极低, 成本高, 容量低, 充放电曲线多平台, 倍率性能差, 高电位下循环性能差 |
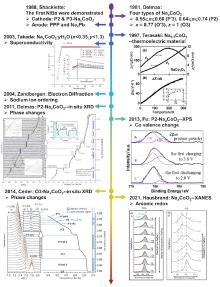



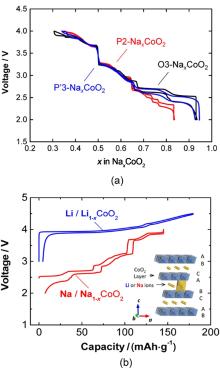
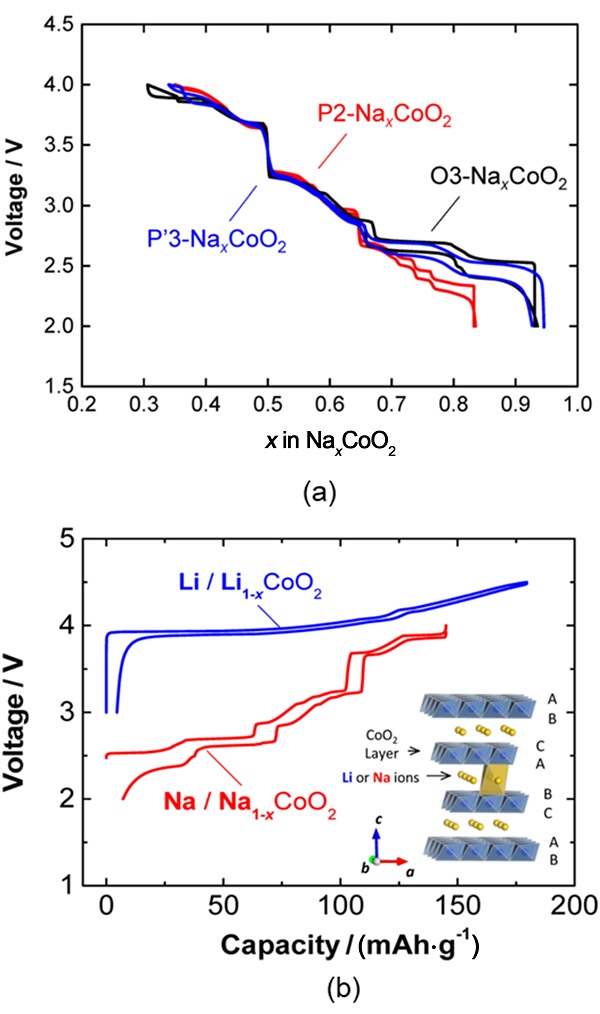
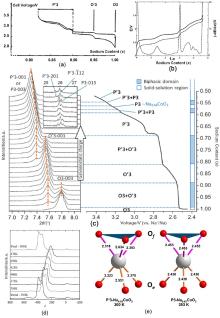





| [1] |
Fang, Z.; Cao, Y.-L.; Hu, Y.-S.; Chen, L.-Q.; Huang, X.-J. Energy Storage Sci. Technol. 2016, 5, 149. (in Chinese)
|
|
(方铮, 曹余良, 胡勇胜, 陈立泉, 黄学杰, 储能科学与技术, 2016, 5, 149.)
|
|
| [2] |
Christoph, V.; Daniel, B.; Marcel, W.; Stefano, P. Nat. Rev. Mater. 2018, 3, 1.
doi: 10.1038/s41578-018-0013-z |
| [3] |
Li, H.; Wu, C.; Wu, F.; Bai, Y. Acta Chim. Sinica 2014, 72, 21. (in Chinese)
doi: 10.6023/A13080830 |
|
(李慧, 吴川, 吴锋, 白莹, 化学学报, 2014, 72, 21.)
doi: 10.6023/A13080830 |
|
| [4] |
Mizushima, K.; Jones, P. C.; Wiseman, P. J.; Goodenough, J. B. Solid State Ionics 1981, 3-4, 171.
doi: 10.1016/0167-2738(81)90077-1 |
| [5] |
Kikkawa, S.; Miyazaki, S.; Koizumi, M. J. Solid State Chem. 1986, 62, 35.
doi: 10.1016/0022-4596(86)90213-6 |
| [6] |
Li, W.; Liu, X.; Celio, H.; Smith, P.; Dolocan, A.; Chi, M.; Manthiram, A. Adv. Energy Mater. 2018, 8, 1703154.
doi: 10.1002/aenm.v8.15 |
| [7] |
Noh, H.-J; Youn, S; Yoon, C. S; Sun, Y.-K. J. Power Sources 2013, 233, 121.
doi: 10.1016/j.jpowsour.2013.01.063 |
| [8] |
Rossouw, M. H.; Thackeray, M. M. Mater. Res. Bull. 1991, 26, 463.
doi: 10.1016/0025-5408(91)90186-P |
| [9] |
Balsys, R. J.; Lindsay Davis, R. Solid State Ionics 1997, 93, 279.
doi: 10.1016/S0167-2738(96)00557-7 |
| [10] |
Delmas, C.; Braconnier, J.; Fouassier, C.; Hagenmuller, P. Solid State Ionics 1981, 3-4, 165.
doi: 10.1016/0167-2738(81)90076-X |
| [11] |
Molenda, J.; Delmas, C.; Hagenmuller, P. Solid State Ionics 1983, 9-10, 431.
doi: 10.1016/0167-2738(83)90271-0 |
| [12] |
Shacklette, L. W.; Jow, T. R.; Townsend, L. J. Electrochem. Soc. 1988, 135, 2669.
doi: 10.1149/1.2095407 |
| [13] |
Shacklette, L. W.; Jow, T. R.; Maxfield, M.; Hatami, R. Synthetic Met. 1989, 28, 655.
doi: 10.1016/0379-6779(89)90586-9 |
| [14] |
Fouassier, C.; Matejka, G.; Reau, J.-M.; Hagenmuller, P. J. Solid State Chem. 1973, 6, 532.
doi: 10.1016/S0022-4596(73)80011-8 |
| [15] |
Liu, L.-L.; Qi, X.-G.; Hu, Y.-S.; Chen, L.-Q.; Huang, X.-J. Acta Chim. Sinica 2017, 75, 218. (in Chinese)
doi: 10.6023/A16080424 |
|
(刘丽露, 戚兴国, 胡勇胜, 陈立泉, 黄学杰, 化学学报, 2017, 75, 218.)
doi: 10.6023/A16080424 |
|
| [16] |
Gutierrez, A.; Dose, W. M.; Borkiewicz, O.; Guo, F.; Avdeev, M.; Kim, S.; Fister, T. T.; Ren, Y.; Bareño, J.; Johnson, C. S. J. Phys. Chem. C 2018, 122, 23251.
doi: 10.1021/acs.jpcc.8b05537 |
| [17] |
Lu, Z.; Dahn, J. R. J. Electrochem. Soc. 2001, 148, A1225.
doi: 10.1149/1.1407247 |
| [18] |
Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Shuji Hitomi; Ryoichi Okuyama; Ryo Usui; Yasuhiro Yamada; Shinichi Komaba. Nat. Mater. 2012, 11, 512.
doi: 10.1038/nmat3309 pmid: 22543301 |
| [19] |
Wu, D.; Li, X.; Xu, B.; Twu, N.; Liu, L.; Ceder, G. Energy Environ. Sci. 2014, 8, 195.
doi: 10.1039/C4EE03045A |
| [20] |
Kumakura, S.; Tahara, Y.; Sato, S.; Kubota, K.; Komaba, S. Chem. Mater. 2017, 29, 8958.
doi: 10.1021/acs.chemmater.7b02772 |
| [21] |
Didier, C.; Guignard, M.; Denage, C.; Szajwaj, O.; Ito, S.; Saadoune, I.; Darriet, J.; Delmas, C. Electrochem. Solid-State Lett. 2011, 14, A75.
doi: 10.1149/1.3555102 |
| [22] |
Yu, C.-Y.; Park, J.-S.; Jung, H.-G.; Chung, K.-Y.; Aurbach, D.; Sun, Y.-K.; Myung, S.-T. Energy Environ. Sci. 2015, 8, 2019.
doi: 10.1039/C5EE00695C |
| [23] |
Caballero, A.; Hernán, L.; Morales, J.; Sánchez, L.; Santos Peña, J.; Aranda, M. A. G. J. Mater. Chem. 2002, 12, 1142.
|
| [24] |
Mendiboure, A.; Delmas, C.; Hagenmuller, P. J. Solid State Chem. France 1985, 57, 323.
doi: 10.1016/0022-4596(85)90194-X |
| [25] |
Kikkawa, S.; Miyazaki, S.; Koizumi, M. Mater. Res. Bull. 1985, 20, 373.
doi: 10.1016/0025-5408(85)90003-0 |
| [26] |
Wang, L.; Wang, J.; Zhang, X.; Ren, Y.; Zuo, P.; Yin, G.; Wang, J. Nano Energy 2017, 34, 215.
doi: 10.1016/j.nanoen.2017.02.046 |
| [27] |
Liu, X.; Zuo, W.; Zheng, B.; Xiang, Y.; Zhou, K.; Xiao, Z.; Shan, P.; Shi, J.; Li, Q.; Zhong, G.; Fu, R.; Yang, Y. Angew. Chem. Int. Ed. 2019, 58, 18086.
doi: 10.1002/anie.v58.50 |
| [28] |
Zuo, W.; Qiu, J.; Liu, X.; Ren, F.; Liu, H.; He, H.; Luo, C.; Li, J.; Ortiz, G. F.; Duan, H.; Liu, J.; Wang, M.-S.; Li, Y.; Fu, R.; Yang, Y. Nat. Commun. 2020, 11, 3544.
doi: 10.1038/s41467-020-17290-6 |
| [29] |
Zuo, W.; Qiu, J.; Liu, X.; Zheng, B.; Zhao, Y.; Li, J.; He, H.; Zhou, K.; Xiao, Z.; Li, Q.; Ortiz, G. F.; Yang, Y. Energy Storage Mater. 2020, 26, 503.
|
| [30] |
Zuo, W.; Ren, F.; Li, Q.; Wu, X.; Fang, F.; Yu, X.; Li, H.; Yang, Y. Nano Energy 2020, 78, 105285.
doi: 10.1016/j.nanoen.2020.105285 |
| [31] |
Fang, Y.-J.; Chen, C.-X.; Ai, X.-P.; Yang, H.-X.; Cao, Y.-L. Acta Phys.-Chim. Sin. 2016, 33, 211. (in Chinese)
doi: 10.3866/PKU.WHXB201610111 |
|
(方永进, 陈重学, 艾新平, 杨汉西, 曹余良, 物理化学学报, 2016, 33, 211.)
|
|
| [32] |
Mizushima, K.; Jones, P. C.; Wiseman, P. J.; Goodenough, J. B. Mater. Res. Bull. 1980, 15, 783.
doi: 10.1016/0025-5408(80)90012-4 |
| [33] |
Hertz, J. T.; Huang, Q.; McQueen, T.; Klimczuk, T.; Bos, J. W. G.; Viciu, L.; Cava, R. J. Phys. Rev. B 2008, 77, 75119.
doi: 10.1103/PhysRevB.77.075119 |
| [34] |
Wang, Z.; Wang, Z.; Peng, W.; Guo, H.; Li, X.; Wang, J.; Qi, A. Ionics 2014, 20, 1525.
doi: 10.1007/s11581-014-1098-z |
| [35] |
Takada, K.; Sakurai, H.; Takayama-Muromachi, E.; Izumi, F.; Dilanian, R. S. T. Nature 2003, 34, 53.
|
| [36] |
Berthelot, R.; Carlier, D.; Delmas, C. Nat. Mater. 2011, 10, 74.
doi: 10.1038/nmat2920 pmid: 21151162 |
| [37] |
Lei, Y.; Li, X.; Liu, L.; Ceder, G. Chem. Mater. 2014, 26, 5288.
doi: 10.1021/cm5021788 |
| [38] |
Terasaki, I.; Sasago, Y.; Uchinokura, K. Phys. Rev. B 1997, 56, 75397.
|
| [39] |
Ding, J. J.; Zhou, Y. N.; Sun, Q.; Yu, X. Q.; Yang, X. Q.; Fu, Z. W. Electrochim. Acta 2013, 87, 388.
doi: 10.1016/j.electacta.2012.09.058 |
| [40] |
Guhl, C.; Rohrer, J.; Kehne, P.; Ferber, T.; Alff, L.; Albe, K.; Jaegermann, W.; Komissinskiy, P.; Hausbrand, R. Energy Storage Mater. 2021, 37, 190.
|
| [41] |
Delmas, C.; Fouassier, C.; Hagenmuller, P. Physica B+C 1980, 99, 81.
doi: 10.1016/0378-4363(80)90214-4 |
| [42] |
Blangero, M.; Carlier, D.; Pollet, M.; Darriet, J.; Delmas, C.; Doumerc, J.-P. Phys. Rev. B 2008, 77, 184116.
doi: 10.1103/PhysRevB.77.184116 |
| [43] |
Han, S. C.; Lim, H.; Jeong, J.; Ahn, D.; Park, W. B.; Sohn, K.-S.; Pyo, M. J. Power Sources 2015, 277, 9.
doi: 10.1016/j.jpowsour.2014.11.150 |
| [44] |
Assadi, M. H. N.; Katayama-Yoshida, H. Comp. Mater. Sci. 2015, 109, 308.
doi: 10.1016/j.commatsci.2015.07.043 |
| [45] |
Bianchini, M.; Wang, J.; Clément, R.; Ceder, G. Adv. Energy Mater. 2018, 8, 1801446.
doi: 10.1002/aenm.v8.26 |
| [46] |
Hasegawa, H.; Ishado, Y.; Okada, S.; Mizuhata, M.; Maki, H.; Matsui, M. J. Electrochem. Soc. 2021, 168, 10509.
doi: 10.1149/1945-7111/abd451 |
| [47] |
Yoshida, H.; Yabuuchi, N.; Komaba, S. Electrochem. Commun. 2013, 34, 60.
doi: 10.1016/j.elecom.2013.05.012 |
| [48] |
Kang, S. M.; Park, J.-H.; Jin, A.; Jung, Y. H.; Mun, J.; Sung, Y.-E. ACS Appl. Mater. Interfaces 2018, 10, 3562.
doi: 10.1021/acsami.7b16077 |
| [49] |
Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Chem. Rev. 2014, 114, 11636.
doi: 10.1021/cr500192f pmid: 25390643 |
| [50] |
Carlier, D.; van der Ven, A.; Delmas, C.; Ceder, G. Chem. Mater. 2003, 15, 2651.
doi: 10.1021/cm030002t |
| [51] |
Liu, Y.-C.; Chen, C.-J.; Zhang, N.; Xiang, X.-D.; Chen, J. J. Electrochem. 2016, 22, 437. (in Chinese)
|
|
(刘永畅, 陈程成, 张宁, 王刘彬, 向兴德, 陈军, 电化学, 2016, 22, 437.)
|
|
| [52] |
Nayak, P. K.; Yang, L.; Brehm, W.; Adelhelm, P. Angew. Chem. Int. Ed. 2018, 57, 102.
|
| [53] |
Biecher, Y.; Smiley, D. L.; Guignard, M.; Fauth, F.; Berthelot, R.; Delmas, C.; Goward, G. R.; Carlier, D. Inorg. Chem. 2020, 59, 5339.
doi: 10.1021/acs.inorgchem.9b03417 pmid: 32250599 |
| [54] |
Liu, H.-Q.; Gao, X.; Chen, J.; Yin, S.-Y.; Zou, K.-Y.; Xu, L.-Q.; Zou, G.-Q.; Hou, H.-S.; Ji, X.-B. Energy Storage Sci. Technol. 2020, 9, 1327. (in Chinese)
|
|
(刘欢庆, 高旭, 陈军, 尹首懿, 邹康宇, 徐来强, 邹国强, 侯红帅, 纪效波, 储能科学与技术, 2020, 9, 1327.)
|
|
| [55] |
Zhu, X.-J.; Zhuang, Y.-H.; Zhao, Y.; Ni, M.-Z.; Xu, J.; Xia, H. Energy Storage Sci. Technol. 2020, 9, 1340. (in Chinese)
|
|
(朱晓辉, 庄宇航, 赵旸, 倪明珠, 徐璟, 夏晖, 储能科学与技术, 2020, 9, 1340.)
|
|
| [56] |
Kubota, K.; Kumakura, S.; Yoda, Y.; Kuroki, K.; Komaba, S. Adv. Energy Mater. 2018, 8, 1703415.
doi: 10.1002/aenm.v8.17 |
| [57] |
Yan, P.-F.; Zheng, J.-M.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Nat. Commun. 2017, 8, 1.
doi: 10.1038/s41467-016-0009-6 |
| [58] |
Carlier, D.; Blangero, M.; Ménétrier, M.; Pollet, M.; Doumerc, J.-P.; Delmas, C. Inorg. Chem. 2009, 48, 7018.
doi: 10.1021/ic900026c pmid: 19419150 |
| [59] |
Rai, A. K.; Anh, L. T.; Gim, J.; Mathew, V.; Kim, J. Ceram. Int. 2014, 40, 2411.
doi: 10.1016/j.ceramint.2013.08.013 |
| [60] |
Shu, G. J.; Chou, F. C. Phys. Rev. B 2008, 78.
|
| [61] |
Shibata, T.; Kobayashi, W.; Moritomo, Y. Appl. Phys. Express 2015, 8, 29202.
doi: 10.7567/APEX.8.029202 |
| [62] |
Willis, T. J.; Porter, D. G.; Voneshen, D. J.; Uthayakumar, S.; Demmel, F.; Gutmann, M. J.; Roger, M.; Refson, K.; Goff, J. P. Sci. Rep. 2018, 8, 3210.
doi: 10.1038/s41598-018-21354-5 pmid: 29453391 |
| [63] |
Hilgenkamp, H.; Ariando
doi: 10.1038/nature01442 |
| [64] |
Foo, M. L.; Wang, Y.; Watauchi, S.; Zandbergen, H. W.; He, T.; Cava, R. J.; Ong, N. P. Phys. Rev. Lett. 2004, 92, 247001.
doi: 10.1103/PhysRevLett.92.247001 |
| [65] |
Mukhamedshin, I. R.; Alloul, H.; Collin, G.; Blanchard, N. Phys. Rev. Lett. 2004, 93, 167601.
pmid: 15525032 |
| [66] |
Mukhamedshin, I. R.; Dooglav, A. V.; Krivenko, S. A.; Alloul, H. Phys. Rev. B 2014, 90, 115151.
doi: 10.1103/PhysRevB.90.115151 |
| [67] |
Platova, T. A.; Mukhamedshin, I. R.; Alloul, H.; Dooglav, A. V.; Collin, G. Phys. Rev. B 2009, 80, 224106.
doi: 10.1103/PhysRevB.80.224106 |
| [68] |
Platova, T. A.; Mukhamedshin, I. R.; Dooglav, A. V.; Alloul, H. JETP Lett. 2010, 91, 421.
doi: 10.1134/S0021364010080126 |
| [69] |
Mukhamedshin, I. R.; Alloul, H. Physica B: Condensed Matter 2015, 460, 58.
doi: 10.1016/j.physb.2014.11.040 |
| [70] |
Zandbergen, H. W.; Foo, M.; Xu, Q.; Kumar, V.; Cava, R. J. Phys. Rev. B 2004, 70, 24101.
doi: 10.1103/PhysRevB.70.024101 |
| [71] |
Chou, F. C.; Chu, M.-W.; Shu, G. J.; Huang, F.-T.; Pai, W. W.; Sheu, H. S.; Lee, P. A. Phys. Rev. Lett. 2008, 101, 127404.
pmid: 18851411 |
| [72] |
Roger, M.; Morris, D. J. P.; Tennant, D. A.; Gutmann, M. J.; Goff, J. P.; Hoffmann, J.-U.; Feyerherm, R.; Dudzik, E.; Prabhakaran, D.; Boothroyd, A. T.; Shannon, N.; Lake, B.; Deen, P. P. Nature 2007, 445, 631.
doi: 10.1038/nature05531 |
| [73] |
Qu, J. F.; Wang, W.; Chen, Y.; Li, G.; Li, X. G. Phys. Rev. B 2006, 73, 250.
|
| [74] |
Chen, J. Acta Phys.-Chim. Sin. 2018, 35, 347. (in Chinese)
doi: 10.3866/PKU.WHXB201804021 |
|
(陈军, 物理化学学报, 2018, 35, 347.)
|
|
| [75] |
de Groot, F. M. F.; Grioni, M.; Fuggle, J. C.; Ghijsen, J.; Sawatzky, G. A.; Petersen, H. Phys. Rev. B 1989, 40, 5715.
pmid: 9992609 |
| [76] |
Valkeapaa, M.; Katsumata, Y.; Asako, I.; Motohashi, T.; Chan, T. S.; Liu, R. S.; Chen, J. M.; Yamauchi, H.; Karppinen, M. J. Solid State Chem. 2007, 180, 1608.
doi: 10.1016/j.jssc.2007.03.001 |
| [77] |
Wang, P.-F.; Yao, H.-R.; Liu, X.-Y.; Yin, Y.-X.; Zhang, J.-N.; Wen, Y.; Yu, X.; Gu, L.; Guo, Y.-G. Sci. Adv. 2018, 4, eaar6018.
doi: 10.1126/sciadv.aar6018 |
| [1] | 王晓, 王星文, 肖乐辉. 单分子荧光成像研究单颗粒纳米催化机制[J]. 化学学报, 2023, 81(8): 1002-1014. |
| [2] | 何家伟, 焦柳, 程雪怡, 陈光海, 吴强, 王喜章, 杨立军, 胡征. 金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究[J]. 化学学报, 2022, 80(7): 896-902. |
| [3] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [4] | 薛晓兰, 张洋, 石美瑜, 李天琳, 黄天龙, 戚继球, 委福祥, 隋艳伟, 金钟. 有机电极材料在非水系金属镁二次电池中的研究进展[J]. 化学学报, 2022, 80(12): 1618-1628. |
| [5] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| [6] | 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684. |
| [7] | 林碧霞, 黄颖珊, 陈帅, 邢震宇. 钠硒电池关键材料的研究进展[J]. 化学学报, 2021, 79(5): 641-648. |
| [8] | 马慧, 张桓荣, 薛面起. 水系钠离子电池的研究进展及实用化挑战[J]. 化学学报, 2021, 79(4): 388-405. |
| [9] | 张璐, 王文凤, 张洪明, 韩树民, 王利民. 水系锌离子电池研究进展和挑战[J]. 化学学报, 2021, 79(2): 158-175. |
| [10] | 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强. α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究[J]. 化学学报, 2021, 79(2): 200-207. |
| [11] | 高霞, 潘会宾, 贺曾贤, 杨柯, 乔成芳, 刘永亮, 周春生. Al-MOF基多级孔Al2O3固定化酶反应器的构筑及构效关系研究[J]. 化学学报, 2021, 79(12): 1502-1510. |
| [12] | 董瑞琪, 吴锋, 白莹, 吴川. 钠离子电池硬碳负极储钠机理及优化策略[J]. 化学学报, 2021, 79(12): 1461-1476. |
| [13] | 梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平. 亚微米去顶角八面体LiNi0.08Mn1.92O4正极材料制备及高温电化学性能[J]. 化学学报, 2021, 79(12): 1526-1533. |
| [14] | 刘庆琳, 任保轶, 孙亚光, 解令海, 黄维. 螺芳基钙钛矿太阳能电池空穴传输材料研究进展[J]. 化学学报, 2021, 79(10): 1181-1196. |
| [15] | 封博谞, 庄小东. 碳基介熵材料:理论与实验[J]. 化学学报, 2020, 78(9): 833-847. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||