Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (4): 485-493.DOI: 10.6023/A21120600 Previous Articles Next Articles
Article
陈守潇a, 刘君珂a, 郑伟琛b, 魏国祯c, 周尧a, 李君涛a,*( )
)
投稿日期:2021-12-30
发布日期:2022-04-28
通讯作者:
李君涛
基金资助:
Shouxiao Chena, Junke Liua, Weichen Zhengb, Guozhen Weic, Yao Zhoua, Juntao Lia( )
)
Received:2021-12-30
Published:2022-04-28
Contact:
Juntao Li
Supported by:Share
Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance[J]. Acta Chimica Sinica, 2022, 80(4): 485-493.


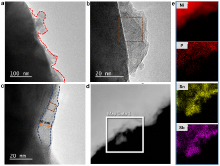

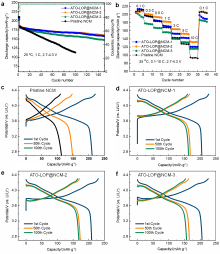



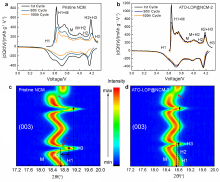


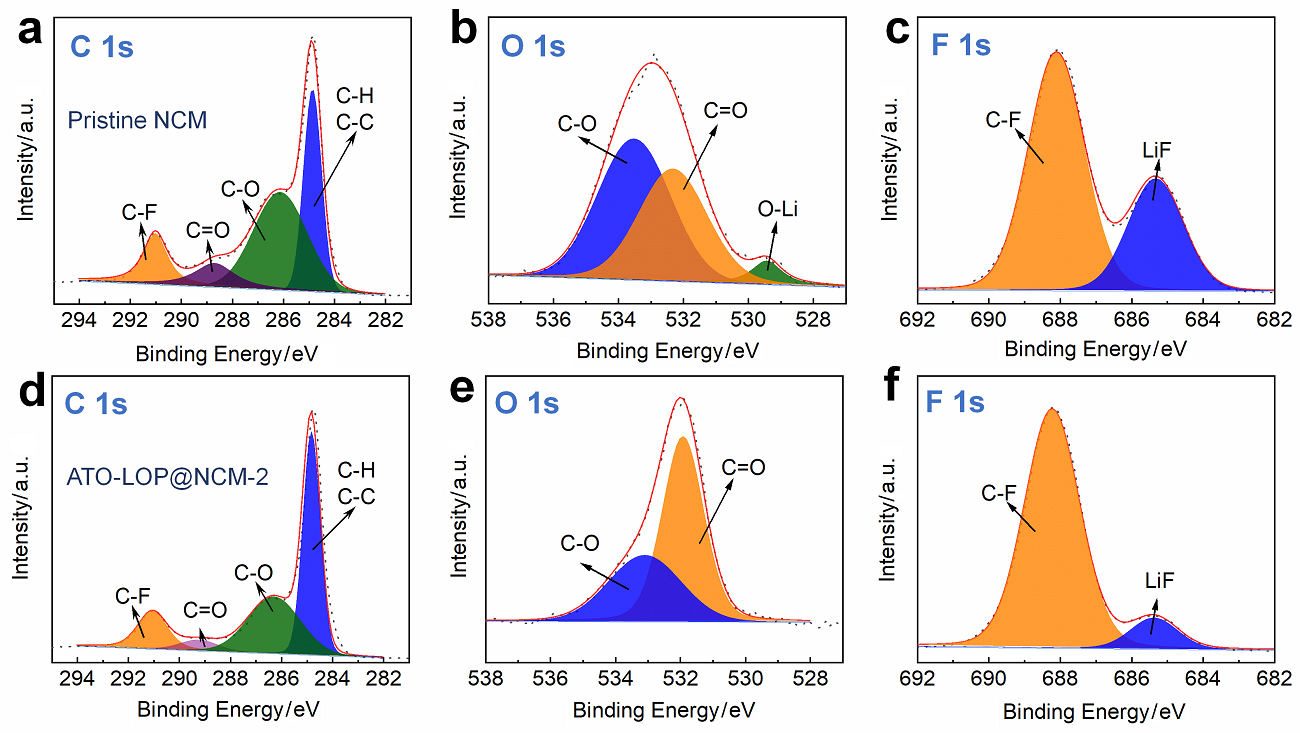
| [1] |
Chen, J. Acta Chim. Sinica 2017, 75, 127. (in Chinese)
doi: 10.6023/A1702E001 |
|
(陈军, 化学学报, 2017, 75, 127.)
doi: 10.6023/A1702E001 |
|
| [2] |
Goodenough, J. B.; Kim, Y. Chem. Mater. 2010, 22, 587.
doi: 10.1021/cm901452z |
| [3] |
Whittingham, M. S. Chem. Rev. 2004, 104, 4271.
pmid: 15669156 |
| [4] |
Zhou, H.; Xin, F.; Pei, B.; Whittingham, M. S. ACS Energy Lett. 2019, 4, 1902.
doi: 10.1021/acsenergylett.9b01236 |
| [5] |
Xu, P.; Zhang, X.-H.; Ma, E.; Rao, F.; Liu, C-W.; Yao, P.-F.; Sun, Z.; Wang, J.-W. Acta Chim. Sinica 2021, 79, 1073. (in Chinese)
doi: 10.6023/A21030083 |
|
(徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟, 化学学报, 2021, 79, 1073.)
doi: 10.6023/A21030083 |
|
| [6] |
Xia, L.; Yu, L.-P.; Hu, D.; Chen, Z. Acta Chim. Sinica 2017, 75, 1183. (in Chinese)
doi: 10.6023/A17060284 |
|
(夏兰, 余林颇, 胡笛, 陈政, 化学学报, 2017, 75, 1183.)
doi: 10.6023/A17060284 |
|
| [7] |
Yang, X.; Lin, M.; Zheng, G.; Wu, J.; Wang, X.; Ren, F.; Zhang, W.; Liao, Y.; Zhao, W.; Zhang, Z.; Xu, N.; Yang, W.; Yang, Y. Adv. Funct. Mater. 2020, 30, 2004664.
doi: 10.1002/adfm.202004664 |
| [8] |
Deng, B.-W.; Sun, D.-M.; Wan, Q.; Wang, H.; Chen, T.; Li, X.; Zhai, M. Z.; Peng, G.-C. Acta Chim. Sinica 2018, 76, 259. (in Chinese)
doi: 10.6023/A17110517 |
|
(邓邦为, 孙大明, 万琦, 王昊, 陈滔, 李璇, 瞿美臻, 彭工厂, 化学学报, 2018, 76, 259.)
doi: 10.6023/A17110517 |
|
| [9] |
Qiu, K.; Yan, M.-X.; Zhao, S.-W.; An, S.-L.; Wang, W.; Jia, G.-X. Acta Chim. Sinica 2021, 79, 1146. (in Chinese)
doi: 10.6023/A21040178 |
|
(邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄, 化学学报, 2021, 79, 1146.)
doi: 10.6023/A21040178 |
|
| [10] |
Xin, F.; Zhou, H.; Zong, Y.; Zuba, M.; Chen, Y.; Chernova, N. A.; Bai, J.; Pei, B.; Goel, A.; Rana, J.; Wang, F.; An, K.; Piper, L. F. J.; Zhou, G.; Whittingham, M. S. ACS Energy Lett. 2021, 6, 1377.
|
| [11] |
Xiao, Y.; Miara, L. J.; Wang, Y.; Ceder, G. Joule 2019, 3, 1252.
doi: 10.1016/j.joule.2019.02.006 |
| [12] |
Lin, H.-C.; Yang, Y. Acta Chim. Sinica 2009, 67, 104. (in Chinese)
|
|
(林和成, 杨勇, 化学学报, 2009, 67, 104.)
|
|
| [13] |
Liu, J.-D.; Zhang, Y.-D.; Liu, J.-X.; Li, J.-H.; Qiu, X.-G.; Cheng, F.-Y. Acta Chim. Sinica 2020, 78, 1426. (in Chinese)
doi: 10.6023/A20070330 |
|
(刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益, 化学学报, 2020, 78, 1426.)
doi: 10.6023/A20070330 |
|
| [14] |
Ren, X.-Q.; Li, D.-L.; Zhao, Z.-Z.; Chen, G.-Q.; Zhao, K.; Kong, X.-Z.; Li, T.-X. Acta Chim. Sinica 2020, 78, 1268. (in Chinese)
doi: 10.6023/A20070319 |
|
(任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心, 化学学报, 2020, 78, 1268)
doi: 10.6023/A20070319 |
|
| [15] |
Liu, H.-H.; Zhang, J.; Lou, Y.-W.; Xia, B.-J. Acta Chim. Sinica 2012, 70, 1055. (in Chinese)
doi: 10.6023/A1112222 |
|
(刘浩涵, 张建, 娄豫皖, 夏保佳, 化学学报, 2012, 70, 1055.)
doi: 10.6023/A1112222 |
|
| [16] |
Kong, J.-Z.; Ren, C.; Tai, G.-A.; Zhang, X.; Li, A.-D.; Wu, D.; Li, H.; Zhou, F. J. Power Sources 2014, 266, 433.
doi: 10.1016/j.jpowsour.2014.05.027 |
| [17] |
Myung, S.-T.; Izumi, K.; Komaba, S.; Yashiro, H.; Bang, H. J.; Sun, Y.-K.; Kumagai, N. J. Phys. Chem. C 2007, 111, 4061.
doi: 10.1021/jp0674367 |
| [18] |
Lu, Y.; Wang, J.-F.; Xie, H.-Q. Acta Chim. Sinica 2021, 79, 1058. (in Chinese)
doi: 10.6023/A21050213 |
|
(陆远, 王继芬, 谢华清, 化学学报, 2021, 79, 1058.)
doi: 10.6023/A21050213 |
|
| [19] |
Li, T.-X.; Li, D.-L.; Zhang, Q.-B.; Gao, J.-H.; Kong, X.-Z.; Fan, X.-Y.; Gou, L. Acta Chim. Sinica 2021, 79, 678. (in Chinese)
doi: 10.6023/A21010019 |
|
(李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾, 化学学报, 2021, 79, 678.)
doi: 10.6023/A21010019 |
|
| [20] |
Chang, Z.; Qiao, Y.; Yang, H.-Z.; Deng, H.; Zhu, X.-Y.; He, P.; Zhou, H.-S. Acta Chim. Sinica 2021, 79, 139. (in Chinese)
doi: 10.6023/A20090442 |
|
(常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎, 化学学报, 2021, 79, 139.)
doi: 10.6023/A20090442 |
|
| [21] |
Liang, Q.-M.; Guo, Y.-J.; Guo, J.-M.; Xiang, M.-W.; Liu, X.-F.; Bai, W.; Ning, P. Acta Chim. Sinica 2021, 79, 1526. (in Chinese)
doi: 10.6023/A21070324 |
|
(梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平, 化学学报, 2021, 79, 1526.)
doi: 10.6023/A21070324 |
|
| [22] |
Liu, J.; Xu, X.; Hu, R.; Yang, L.; Zhu, M. Adv. Energy Mater. 2016, 6, 1600256.
doi: 10.1002/aenm.201600256 |
| [23] |
Xu, Q.; Li, X.; Sari, H. M. K.; Li, W.; Liu, W.; Hao, Y.; Qin, J.; Cao, B.; Xiao, W.; Xu, Y.; Wei, Y.; Kou, L.; Tian, Z.; Shao, L.; Zhang, C.; Sun, X. Nano Energy, 2020, 77, 105034.
doi: 10.1016/j.nanoen.2020.105034 |
| [24] |
Wang, C.-W.; Zhou, Y.; You, J.-H.; Chen, J.-D.; Zhang, Z.; Zhang, S.-J.; Shi, C.-G.; Zhang, W.-D.; Zou, M.-H.; Yu, Y.; Li, J.-T.; Zeng, L.-Y.; Huang, L.; Sun, S.-G. ACS Appl. Energy Mater. 2020, 3, 2593.
doi: 10.1021/acsaem.9b02291 |
| [25] |
Griffith, K. J.; Wiaderek, K. M.; Cibin, G.; Marbella, L. E.; Grey, C. P. Nature 2018, 559, 556.
doi: 10.1038/s41586-018-0347-0 |
| [26] |
Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T.; Li, M.; Lin, R.; Chen, Y.; Li, Y.; Xing, X.; Choi, Y.; Gao, L.; Cho, H. S.; An, K.; Feng, J.; Kostecki, R.; Amine, K.; Wu, T.; Lu, J.; Xin, H. L.; Ong, S. P.; Liu, P. Nature 2020, 585, 63.
doi: 10.1038/s41586-020-2637-6 |
| [27] |
Guo, B.; Yu, X.; Sun, X.-G.; Chi, M.; Qiao, Z.-A.; Liu, J.; Hu, Y.-S.; Yang, X.-Q.; Goodenoughe, J. B.; Dai, S. Energy Environ. Sci. 2014, 7, 2220.
doi: 10.1039/C4EE00508B |
| [28] |
Ryu, H.-H.; Park, K.-J.; Yoon, D. R.; Aishova, A.; Yoon, C. S.; Sun, Y.-K. Adv. Energy Mater. 2019, 9, 1902698.
doi: 10.1002/aenm.201902698 |
| [29] |
Zhou, A.; Dai, X.; Lu, Y.; Wang, Q.; Fu, M.; Li, J. ACS Appl. Mater. Interfaces 2016, 8, 34123.
doi: 10.1021/acsami.6b11630 |
| [30] |
Zhang, X.; Zhang, X.; Sun, X.; An, Y.; Song, S.; Li, C.; Wang, K.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; Ma, Y. J. Power Sources 2021, 488, 229454.
doi: 10.1016/j.jpowsour.2021.229454 |
| [31] |
Li, J.; Manthiram, A. Adv. Energy Mater. 2019, 9, 1902731.
doi: 10.1002/aenm.201902731 |
| [32] |
Zou, L.; Zhao, W.; Liu, Z.; Jia, H.; Zheng, J.; Wang, G.; Yang, Y.; Zhang, J.-G.; Wang, C. ACS Energy Lett. 2018, 3, 2433.
doi: 10.1021/acsenergylett.8b01490 |
| [33] |
Das, H.; Urban, A.; Huang, W.; Ceder, G. Chem. Mater. 2017, 29, 7840.
doi: 10.1021/acs.chemmater.7b02546 |
| [34] |
Liu, W.; Oh, P.; Liu, X.; Lee, M. J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Angew. Chem. Int. Ed. 2015, 54, 4440.
doi: 10.1002/anie.201409262 |
| [35] |
Zhao, J.; Zhang, W.; Huq, A.; Misture, S. T.; Zhang, B.; Guo, S.; Wu, L.; Zhu, Y.; Chen, Z.; Amine, K.; Pan, F.; Bai, J.; Wang, F. Adv. Energy Mater. 2017, 7, 1601266.
doi: 10.1002/aenm.201601266 |
| [36] |
Kim, U. H.; Ryu, H. H.; Kim, J. H.; Mücke, R.; Kaghazchi, P.; Yoon, C. S.; Sun, Y. K. Adv. Energy Mater. 2019, 9, 1803902.
doi: 10.1002/aenm.201803902 |
| [1] | Xiaoyu Gu, Jin Li, Qian Sun, Chaoyang Wang. Microcalorimetry Analysis of Thermal Runaway Process in Lithium-ion Batteries [J]. Acta Chimica Sinica, 2024, 82(2): 146-151. |
| [2] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [3] | Yalan Zhang, Zhixiang Yuan, Hao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of High-energy-density Solid-state Lithium Ion Batteries Employing Ni-rich Ternary Cathodes [J]. Acta Chimica Sinica, 2023, 81(12): 1724-1738. |
| [4] | Wanying Chang, Yingying Tan, Jingyi Wu, Yingjie Liu, Jinhai Cai, Chunyan Lai. Study on the Properties of Polyethylene Oxide Based Solid State Electrolyte Enhanced by Three-Dimensional Structured Li6.28La3Zr2Al0.24O12 [J]. Acta Chimica Sinica, 2023, 81(12): 1708-1715. |
| [5] | Shuang Zhang, Chengfei Yang, Yubo Yang, Ningning Feng, Gang Yang. Electrochemical Behaviors of LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn) Recycled from Spent Li-ion Batteries as Cathodic Catalyst for Lithium-Oxygen Battery [J]. Acta Chimica Sinica, 2022, 80(9): 1269-1276. |
| [6] | Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 [J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153. |
| [7] | Ping Xu, Xihua Zhang, En Ma, Fu Rao, Chunwei Liu, Peifan Yao, Zhi Sun, Jingwei Wang. Selective Recovery of Lithium from Spent Lithium-ion Batteries Synergized by Carbon and Sulfur Elements [J]. Acta Chimica Sinica, 2021, 79(8): 1073-1081. |
| [8] | Zhi Chang, Yu Qiao, Huijun Yang, Han Deng, Xingyu Zhu, Ping He, Haoshen Zhou. Applications of Metal-organic Frameworks (MOFs) Materials in Lithium-ion Battery/Lithium-metal Battery Electrolytes [J]. Acta Chimica Sinica, 2021, 79(2): 139-145. |
| [9] | Wang Shan, Fan Xiaoyong, Cui Yu, Gou Lei, Wang Xingang, Li Donglin. Three-dimensional Porous Current Collector for Lithium Storage Enhancement of NiO Electrode [J]. Acta Chim. Sinica, 2019, 77(6): 551-558. |
| [10] | Deng Bangwei, Sun Daming, Wan Qi, Wang Hao, Chen Tao, Li Xuan, Qu Meizhen, Peng Gongchang. Review of Electrolyte Additives for Ternary Cathode Lithium-ion Battery [J]. Acta Chim. Sinica, 2018, 76(4): 259-277. |
| [11] | He Qian, Zhang Chong, Li Xiao, Wang Xue, Mu Pan, Jiang Jiaxing. Pyrene-Based Conjugated Microporous Polymer as High Performance Electrode for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2018, 76(3): 202-208. |
| [12] | Li Zhiwei, Zhong Jialiang, Chen Nannan, Xue Bing, Mi Hongyu. Template-Assisted Preparation and Lithium Storage Performance of Nitrogen Doped Porous Carbon Sheets [J]. Acta Chim. Sinica, 2018, 76(3): 209-214. |
| [13] | Zheng Zhuo, Wu Zhenguo, Xiang Wei, Guo Xiaodong. Preparation and Electrochemical Performance of High Rate Spherical Layered LiNi0.5Co0.2Mn0.3O2 Cathode Material for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2017, 75(5): 501-507. |
| [14] | Zhang Guobin, Xiong Tengfei, Pan Xuelei, Yan Mengyu, Han Chunhua, Mai Liqiang. In Situ Observation and Mechanism Investigation of Lattice Breathing in Vanadium Oxide Cathode [J]. Acta Chim. Sinica, 2016, 74(7): 582-586. |
| [15] | Lyu Zhiyang, Feng Rui, Zhao Jin, Fan Hao, Xu Dan, Wu Qiang, Yang Lijun, Chen Qiang, Wang Xizhang, Hu Zheng. Nitrogen-Doped Carbon Nanocages as High-Rate Anode for Lithium Ion Batteries [J]. Acta Chim. Sinica, 2015, 73(10): 1013-1017. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||