Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (4): 438-443.DOI: 10.6023/A21120619 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Communication
江辉波a,b, 林姗姗a,b, 徐玉平b, 孙径b, 徐忠宁b,*( ), 郭国聪b,*(
), 郭国聪b,*( )
)
投稿日期:2021-12-31
发布日期:2022-04-28
通讯作者:
徐忠宁, 郭国聪
作者简介:基金资助:
Huibo Jianga,b, Shanshan Lina,b, Yuping Xub, Jing Sunb, Zhongning Xub( ), Guocong Guob(
), Guocong Guob( )
)
Received:2021-12-31
Published:2022-04-28
Contact:
Zhongning Xu, Guocong Guo
About author:Supported by:Share
Huibo Jiang, Shanshan Lin, Yuping Xu, Jing Sun, Zhongning Xu, Guocong Guo. Lewis Acid in NaY Zeolite High Selectively Catalyze Methanol to Dimethoxymethane via Methyl Nitrite※[J]. Acta Chimica Sinica, 2022, 80(4): 438-443.
| Sample | SBET a/ (m2•g-1) | Vmic b/ (cm3•g-1) | Vmes c/ (cm3•g-1) | Average Dp d/ nm |
|---|---|---|---|---|
| NaY | 918.05 | 0.32 | 0.05 | 4.81 |
| HY | 866.37 | 0.30 | 0.05 | 5.73 |
| HZSM-5 | 324.36 | 0.10 | 0.04 | 6.05 |
| Hβ | 594.81 | 0.17 | 0.29 | 9.15 |
| Sample | SBET a/ (m2•g-1) | Vmic b/ (cm3•g-1) | Vmes c/ (cm3•g-1) | Average Dp d/ nm |
|---|---|---|---|---|
| NaY | 918.05 | 0.32 | 0.05 | 4.81 |
| HY | 866.37 | 0.30 | 0.05 | 5.73 |
| HZSM-5 | 324.36 | 0.10 | 0.04 | 6.05 |
| Hβ | 594.81 | 0.17 | 0.29 | 9.15 |
| Sample | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| MF | DMM | MeOH | ||
| HY | 97 | 69 | 12 | 19 |
| HZSM-5 | 90 | 79 | 3 | 18 |
| Hβ | 89 | 80 | 4 | 16 |
| NaY | 97 | 29 | 53 | 18 |
| NaZSM-5 | 18 | 45 | 7 | 48 |
| Naβ | 6 | 25 | 12 | 63 |
| Sample | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| MF | DMM | MeOH | ||
| HY | 97 | 69 | 12 | 19 |
| HZSM-5 | 90 | 79 | 3 | 18 |
| Hβ | 89 | 80 | 4 | 16 |
| NaY | 97 | 29 | 53 | 18 |
| NaZSM-5 | 18 | 45 | 7 | 48 |
| Naβ | 6 | 25 | 12 | 63 |
| Sample | Lewis acid sites/ (μmol•g -1) | Brönsted acid sites/ (μmol•g -1) |
|---|---|---|
| HY | 71.4 | 210.0 |
| HZSM-5 | 10.7 | 183.9 |
| Hβ | 81.6 | 111.2 |
| NaY | 437.9 | 8.7 |
| NaZSM-5 | 102.4 | 11.3 |
| Naβ | 71.6 | 7.1 |
| Sample | Lewis acid sites/ (μmol•g -1) | Brönsted acid sites/ (μmol•g -1) |
|---|---|---|
| HY | 71.4 | 210.0 |
| HZSM-5 | 10.7 | 183.9 |
| Hβ | 81.6 | 111.2 |
| NaY | 437.9 | 8.7 |
| NaZSM-5 | 102.4 | 11.3 |
| Naβ | 71.6 | 7.1 |
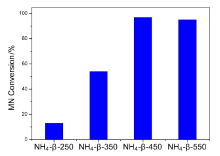


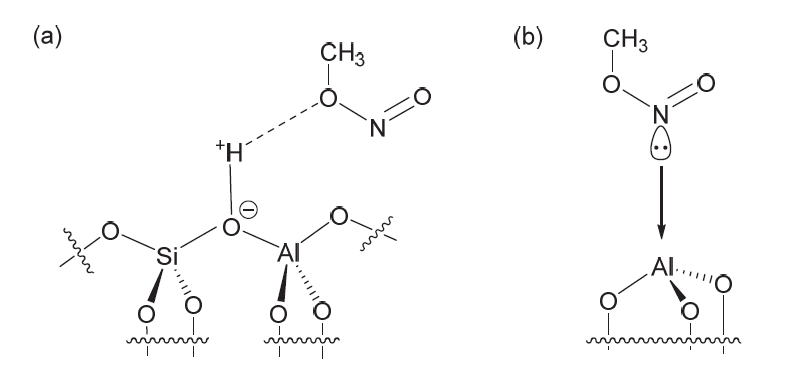
| [1] |
Sun, R.; Delidovich, I.; Palkovits, R. ACS Catal. 2019, 9, 1298.
doi: 10.1021/acscatal.8b04441 |
| [2] |
Ren, Y.; Huang, Z.-H.; Jiang, D.-M.; Liu, L.-X.; Zeng, K.; Liu, B.; Wang, X.-B. P. I. Mech. Eng. D: J. Aut. Eng. 2005, 219, 905.
|
| [3] |
Maricq, M. M.; Chase, R. E.; Podsiadlik, D. H. Siegl, W. O.; Kaiser, E. W. Sae Int. J. Eng. 1998, 107, 1504.
|
| [4] |
Lian, H.-K.; Long, H.-M. Nat. Gas Ind. 2012, 37, 68.
|
| [5] |
Lautenschütz, L.; Oestreich, D.; Seidenspinner, P.; Arnold, U.; Sauer, J. Fuel 2016, 173, 129.
doi: 10.1016/j.fuel.2016.01.060 |
| [6] |
Fu, Y.-C.; Sun, Q.; Shen, J.-Y. Chin. J. Catal. 2009, 30, 791.
doi: 10.1016/S1872-2067(18)30010-X |
| [7] |
Xie, Z.-Q.; Chen, C.-B.; Hou, B.; Sun, D.-K.; Guo, H.-Q.; Wang, J.-G.; Li, D.-B.; Jia, L.-T. J. Phys. Chem. C. 2018, 122, 9909.
doi: 10.1021/acs.jpcc.8b00421 |
| [8] |
Vermeire, F. H.; Carstensen, H. H.; Herbinet, O.; Battin-Leclerc, F.; Marin, G. B.; Van, G.; Kevin, M. Com. Flame. 2018, 190, 270.
|
| [9] |
Liu, Z.-Y.; Zhang, Z.-Y.; Ge, C.-Y.; Wang, H.; Li, L. Chem. Ind. Times. 2014, 1, 27. (in Chinese)
|
|
(刘震宇, 张中宇, 葛常艳, 王辉, 李磊, 化工时刊, 2014, 1, 27.)
|
|
| [10] |
Tatibouët, J. M. Appl. Catal. A Gen. 1997, 148, 213.
doi: 10.1016/S0926-860X(96)00236-0 |
| [11] |
Zhuo, G.-L.; Jiang, X.-Z. Catal. Lett. 2002, 2, 219.
doi: 10.1007/BF00766210 |
| [12] |
Li, Z.-H.; Wang, W.-H.; Yin, D.-X.; Lv, J.; Ma, X.-B. Front. Chem. Sci. Eng. 2012, 6, 410.
doi: 10.1007/s11705-012-1213-5 |
| [13] |
Xu, Z.-N.; Sun, J.; Lin, C.-S.; Jiang, X.-M.; Chen, Q.-S.; Peng, S.-Y.; Wang, M.-S.; Guo, G.-C. ACS Catal. 2013, 3, 118.
doi: 10.1021/cs300759h |
| [14] |
Peng, S.-Y.; Xu, Z.-N.; Chen, Q.-S.; Chen, Y.-M.; Sun, J.; Wang, Z.-Q.; Wang, M.-S.; Guo, G.-C. Chem. Commun. 2013, 49, 5718.
doi: 10.1039/c3cc00219e |
| [15] |
Peng, S.-Y.; Xu, Z.-N.; Chen, Q.-S.; Wang, Z.-Q.; Chen, Y.-M.; Lv, D.-M.; Lu, G.; Guo, G.-C. Catal. Sci. Tech. 2014, 4, 1925.
doi: 10.1039/C4CY00245H |
| [16] |
Peng, S.-Y.; Xu, Z.-N.; Chen, Q.-S.; Wang, Z.-Q.; Lv, D.-M.; Sun, J.; Chen, Y.-M.; Guo, G.-C. ACS Catal. 2015, 5, 4410.
doi: 10.1021/acscatal.5b00365 |
| [17] |
Wang, Z.-Q.; Sun, J.; Xu, Z.-N.; Guo, G.-C. Nanoscale 2020, 12, 20131.
doi: 10.1039/D0NR03008B |
| [18] |
Jing, K.-Q.; Fu, Y.-Q.; Chen, Z.-N.; Zhang, T.; Sun, J.; Xu, Z.-N.; Guo, G.-C. ACS Appl. Mater. Inter. 2021, 13, 24856.
doi: 10.1021/acsami.1c04523 |
| [19] |
Jing, K.-Q.; Fu, Y.-Q.; Wang, Z.-Q.; Chen, Z.-N.; Guo, G.-C. Nanoscale 2020, 12, 27.
|
| [20] |
Zhang, M.-T.; Yan, T.-T.; Dai, W.-L.; Guan, N.-J.; Li, L.-D. Acta Chim. Sinica 2020, 78, 1404. (in Chinese)
doi: 10.6023/A20080346 |
|
(张梦婷, 颜婷婷, 戴卫理, 关乃佳, 李兰冬, 化学学报,, 2020, 78, 1404.)
doi: 10.6023/A20080346 |
|
| [21] |
Yao, X.-T.; Huang, X.; Lin, Y.-X.; Liu, Y.-M. Acta Chim. Sinica 2020, 78, 1111. (in Chinese)
doi: 10.6023/A20060246 |
|
(姚旭婷, 黄鑫, 林玉霞, 刘月明, 化学学报,, 2020, 78, 1111.)
doi: 10.6023/A20060246 |
|
| [22] |
Li, Y.-C.; Wang, H.; Dong, M.; Li, J.-F.; Wang, G.-F.; Qin, Z.-F.; Fan, W.-B.; Wang, J.-G. Acta Chim. Sinica 2016, 74, 529. (in Chinese)
doi: 10.6023/A16020077 |
|
(李艳春, 王浩, 董梅, 李俊汾, 王国富, 秦张峰, 樊卫斌, 王建国, 化学学报,, 2016, 74, 529.)
doi: 10.6023/A16020077 |
|
| [23] |
Chung, K.-H.; Park, B.-G. J. Ind. Eng. Chem. 2009, 15, 388.
doi: 10.1016/j.jiec.2008.11.012 |
| [24] |
Sun, D.; Sun, B.; Pei, Y.; Yan, S.-R.; Fan, K.-N.; Qiao, M.-H.; Zhang, X.-X.; Zong, B.-N. Acta Chim. Sinica 2021, 79, 771. (in Chinese)
doi: 10.6023/A20120563 |
|
(孙冬, 孙博, 裴燕, 闫世润, 范康年, 乔明华, 张晓昕, 宗保宁, 化学学报,, 2021, 79, 771.)
doi: 10.6023/A20120563 |
|
| [25] |
Feng, A.-H.; Yu, Y.; Yu, Y.; Song, L.-X. Acta Chim. Sinica 2018, 76, 17. (in Chinese)
|
|
(冯爱虎, 于洋, 于云, 宋力昕, 化学学报,, 2018, 76, 17.)
|
|
| [26] |
Cheng, S.-J.; Zeng, Y.; Pei, Y.; Fan, K.-N.; Qiao, M.-H.; Zong, B.-N. Acta Chim. Sinica 2019, 77, 1054. (in Chinese)
doi: 10.6023/A19060219 |
|
(成诗婕, 曾杨, 裴燕, 范康年, 乔明华, 宗保宁, 化学学报,, 2019, 77, 1054.)
doi: 10.6023/A19060219 |
|
| [27] |
Zhou, L.-P.; Liu, Z.; Bai, Y.-Q.; Lu, T.-L.; Yang, X.-M.; Xu, J. J. Energy. Chem. 2016, 25, 141.
doi: 10.1016/j.jechem.2015.11.010 |
| [28] |
Celik, F. E.; Kim, T. J.; Bell, A. T. J. Catal. 2010, 270, 185.
doi: 10.1016/j.jcat.2009.12.021 |
| [29] |
Xin, Q. Research Methods of Solid Catalysts (Volume One), Science Press, Beijing, 2004, p. 273. (in Chinese)
|
|
(辛勤, 固体催化剂研究方法(上), 科学出版社, 北京, 2004, p. 273.)
|
|
| [30] |
Choudhary, V. R.; Pataskar, S. G. Mater. Chem. Phys. 1986, 13, 587.
doi: 10.1016/0254-0584(85)90008-2 |
| [31] |
Xin, Q.; Xu, J. Modern Catalytic Chemistry, Science Press, Beijing, 2016, p. 128. (in Chinese)
|
|
(辛勤, 徐杰, 现代催化化学, 科学出版社, 北京, 2016, p. 128.)
|
| [1] | Xupan Xu, Kai Fan, Shengze Zhao, Jian Li, Shan Gao, Zhongbiao Wu, Xiangju Meng, Feng-Shou Xiao. Enhanced Performance for Mesoporous Beta Zeolites Supported Pd in the Methane Catalytic Combustion★ [J]. Acta Chimica Sinica, 2023, 81(9): 1108-1112. |
| [2] | Mei Hong, Jinqiang Gao, Tong Li, Shihe Yang. In-situ Etching Strategy for Manipulation of Hierarchical Zeolite and Its Application★ [J]. Acta Chimica Sinica, 2023, 81(8): 937-948. |
| [3] | Junchang Chen, Mingxing Zhang, Shuao Wang. Research Progress of Synthesis Methods for Crystalline Porous Materials [J]. Acta Chimica Sinica, 2023, 81(2): 146-157. |
| [4] | Hongdan Zhang, Xinyu Lan, Peng Cheng. Advances in Hydroxyl Free Radical Assisted Synthesis of Zeolite [J]. Acta Chimica Sinica, 2023, 81(1): 100-110. |
| [5] | Lei He, Qiuxiang Yao, Ming Sun, Xiaoxun Ma. Progress in Preparation and Catalysis of Two-dimensional (2D) and Three-dimensional (3D) Zeolites [J]. Acta Chimica Sinica, 2022, 80(2): 180-198. |
| [6] | Guo Jinqiu, Du Yaping, Zhang Hongbo. A Brief Summary of Research Progress on the Application of Rare Earth Materials in Heterogeneous Catalysis [J]. Acta Chimica Sinica, 2020, 78(7): 625-633. |
| [7] | Zhang Mengting, Yan Tingting, Dai Weili, Guan Naijia, Li Landong. Zeolite Stabilized Isolated Molybdenum Species for Catalytic Oxidative Desulfurization [J]. Acta Chimica Sinica, 2020, 78(12): 1404-1410. |
| [8] | Wei Simin, Wang Yinghui, Zhao Hongmei. Study on the Mechanism of Frustrated Lewis Pairs Catalysed Hydrogenation of 2,3-Disubstituted 2H-1,4-Benzoxazine [J]. Acta Chimica Sinica, 2019, 77(3): 278-286. |
| [9] | Wang Leming, Wang Qian, Chen Jiean, Huang Yong. Switching Reaction Pathways by Cooperative Catalysis of N-Heterocyclic Carbene and Lewis Acids [J]. Acta Chim. Sinica, 2018, 76(11): 850-856. |
| [10] | Feng Aihu, Yu Yang, Yu Yun, Song Lixin. Recent Progress in the Removal of Volatile Organic Compounds by Zeolite and Its Supported Catalysts [J]. Acta Chim. Sinica, 2018, 76(10): 757-773. |
| [11] | Hu Chengyu, Yan Wenfu, Xu Ruren. Phase Transition Behavior of Zeolite Y under Hydrothermal Conditions [J]. Acta Chim. Sinica, 2017, 75(7): 679-685. |
| [12] | Yang Xiaodong, Wang Xinmiao, Gao Shanbin, Wang Anjie. Hydrodesulfurization Performances of Pd Catalysts Supported on ZSM-5/MCM-41 Composite Zeolite [J]. Acta Chim. Sinica, 2017, 75(5): 479-484. |
| [13] | Li Yanchun, Wang Hao, Dong Mei, Li Junfen, Wang Guofu, Qin Zhangfeng, Fan Weibin, Wang Jianguo. Optimization of Reaction Conditions in the Transalkylation of Toluene with 1,2,4-Trimethylbenzene Catalyzed by Beta Zeolite and the Investigation of Its Reaction Mechanism [J]. Acta Chim. Sinica, 2016, 74(6): 529-537. |
| [14] | Zhang Qichao, Lü Jian, Luo Sanzhong. Carbocation Lewis Acid Catalyzed Redox-Neutral α-C(sp3)H Arylation of Amines [J]. Acta Chim. Sinica, 2016, 74(1): 61-66. |
| [15] | Tan Fen, Chen Jiarong, Wang Ping, Xiao Wenjing. Asymmetric Diels-Alder Reaction of 2-Arylidene-1,3-indanediones with 2-Vinylindoles Catalyzed by a Sc(OTf)3/Bis(oxazoline) Complex:Enantioselective Synthesis of Tetrahydrocarbazole Spiro Indanedione Derivatives [J]. Acta Chimica Sinica, 2014, 72(7): 836-840. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||