有机化学 ›› 2012, Vol. ›› Issue (03): 560-566.DOI: 10.6023/cjoc1109081 上一篇 下一篇
研究论文
蔡双莲a, 吴峥a, 吴进a, 汪秋安a, 单杨b
Cai Shuangliana, Wu Zhenga, Wu Jinga, Wang Qiuana, Shan Yangb
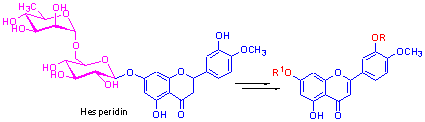
以橙皮苷为原料, 经脱氢、选择性甲基化、糖苷水解、相转移催化下的糖苷化反应、异戊烯基化和法呢烯基化等反应步骤, 分别合成了3'-O-甲基香叶木素(1), 香叶木素-7-O-β-D-葡萄糖苷(2), 香叶木素-7-O-β-D-半乳糖苷(3), 3'-O-甲基香叶木素-7-O-β-D-葡萄糖苷(4) 4 种天然产物及3'-O-甲基香叶木素-7-O-β-D-半乳糖苷(5), 香叶木素-7-O-β-D-乙酰葡萄糖苷(6)、香叶木素-7-O-β-D-乙酰半乳糖苷(7), 3'-O-甲基香叶木素-7-O-β-D-乙酰葡萄糖苷(8), 3'-O-甲基香叶木素-7-O-β-D-乙酰半乳糖苷(9), 7-O-异戊基香叶木素(10), 7-O-异戊烯基-3'-O-甲基香叶木素(11)和7-O-法呢烯基-3'-O-甲基香叶木素(12) 8 种新的香叶木素衍生物. 所合成化合物的结构已由核磁共振谱、红外光谱和质谱所证实, 并用比色法MTT[3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐]蛋白染色法对所合成的目标化合物进行了体外抗肿瘤细胞生物活性测试,发现化合物6, 10 和11 对肝癌细胞(SMMC-7721)、乳腺癌细胞(MCF-7)和结肠癌细胞(SW480)有一定的抑制活性.