有机化学 ›› 2023, Vol. 43 ›› Issue (9): 3196-3209.DOI: 10.6023/cjoc202301020 上一篇 下一篇
研究论文
周然, 袁春梅, 张桃, 毛飘, 刘燚, 孟开妮, 幸惠, 薛伟*( )
)
收稿日期:2023-04-25
修回日期:2023-05-29
发布日期:2023-06-06
基金资助:
Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue( )
)
Received:2023-04-25
Revised:2023-05-29
Published:2023-06-06
Contact:
E-mail: Supported by:文章分享

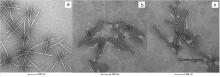
设计合成了25个含喹唑啉酮的查尔酮衍生物. 抗病毒生物实验结果表明, (E)-3-(4-氯苯基)-6-氟-2-((2-(4-(3-(4-甲氧基苯基)丙烯酰基)苯氧基)乙基)硫代)喹唑啉-4(3H)-酮(Z12)对烟草花叶病毒(tobacco mosaic virus, TMV)有较好的抑制活性, 治疗和保护的EC50值分别为94.3和98.8 μg/mL, 优于对照药宁南霉素(ningnanmycin, NNM)的216.1和189.6 μg/mL. 透射电镜结果表明, Z12使得TMV病毒颗粒断裂, 形成长短不一的棒状结构, 微量热涌动实验(MST)的结果表明, Z12对烟草花叶病毒外壳蛋白(TMV-CP)结合的Kd值为(0.033±0.014) μmol/L, 优于宁南霉素的Kd值[(0.106±0.024) μmol/L], 说明了Z12对TMV-CP具有更强的结合力. 抑菌实验结果表明, 部分化合物对水稻白叶枯病菌(Xanthomonas oryzae pv. Oryzae, Xoo)和柑桔溃疡病菌(Xanthomonas axonopodis pv. Citri, Xac)表现出优异活性, 其中(E)-2-((2-(4-(3-(4-溴苯基)丙烯酰基)苯氧基)乙基)硫代)-3-(4-氯苯基)-6-硝基喹唑啉-4(3H)-酮(Z25)对Xoo活性最好, EC50值为19.5 μg/mL, 优于叶枯唑(77.1 μg/mL)和噻菌铜(95.9 μg/mL). 扫描电镜结果表明, Z25作用后使得Xoo的细胞膜皱缩变形. 上述实验结果表明含喹唑啉酮的查尔酮衍生物是有潜力的抗病毒和抑菌药物.
周然, 袁春梅, 张桃, 毛飘, 刘燚, 孟开妮, 幸惠, 薛伟. 含喹唑啉酮的查尔酮衍生物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3196-3209.
Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone[J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209.


| Compd. | Curative activity/% | Protective activity/% | Inactivation activity/% | Compd. | Curative activity/% | Protective activity/% | Inactivation activity/% |
|---|---|---|---|---|---|---|---|
| Z1 | 31.5±4.6 | 17.9±3.4 | 37.5±0.8 | Z19 | 25.1±1.1 | 45.8±0.7 | 53.1±4.2 |
| Z2 | 32.3±2.7 | 56.3±3.0 | 32.3±2.7 | Z20 | 62.5±2.9 | 35.5±1.3 | 47.6±3.2 |
| Z3 | 44.8±0.4 | 45.8±2.5 | 44.8±0.4 | Z21 | 52.3±3.5 | 55.7±2.4 | 58.5±3.3 |
| Z4 | 51.2±2.2 | 35.8±2.5 | 23.0±1.9 | Z22 | 24.7±2.7 | 36.9±0.9 | 64.7±3.9 |
| Z5 | 66.9±3.2 | 67.4±1.1 | 39.8±0.2 | Z23 | 17.9±2.3 | 20.1±1.7 | 38.1±1.6 |
| Z6 | 34.7±2.1 | 44.1±3.4 | 33.6±0.8 | Z24 | 36.6±2.5 | 18.9±2.3 | 28.1±1.2 |
| Z7 | 26.2±2.1 | 71.1±0.9 | 26.2±2.1 | Z25 | 22.2±1.8 | 27.7±2.9 | 54.1±2.8 |
| Z8 | 34.6±2.6 | 55.9±3.1 | 46.8±1.8 | K1 | 25.5±1.1 | 32.1±2.0 | 33.7±1.4 |
| Z9 | 22.8±4.1 | 51.1±0.8 | 26.9±1.8 | K2 | 35.3±2.3 | 27.9±1.5 | 42.1±1.1 |
| Z10 | 45.6±3.7 | 63.0±1.9 | 45.6±3.7 | K3 | 38.7±1.4 | 35.6±2.0 | 23.3±0.9 |
| Z11 | 36.6±4.9 | 23.0±2.9 | 36.6±4.9 | K4 | 27.3±1.2 | 30.1±2.3 | 34.5±1.5 |
| Z12 | 66.6±2.9 | 70.4±2.7 | 69.5±1.3 | K5 | 40.2±2.3 | 34.4±1.7 | 37.5±1.2 |
| Z13 | 35.0±4.1 | 33.7±1.1 | 25.0±4.1 | C1 | 33.9±1.7 | 24.3±2.1 | 35.9±1.3 |
| Z14 | 32.3±1.2 | 34.6±2.2 | 61.5±1.3 | C2 | 29.9±2.0 | 35.8±1.6 | 27.9±2.1 |
| Z15 | 28.7±1.1 | 59.8±2.5 | 31.5±0.9 | C3 | 39.9±1.6 | 32.2±1.4 | 31.1±2.0 |
| Z16 | 19.8±0.8 | 38.9±1.0 | 63.4±2.9 | C4 | 43.3±1.9 | 33.2±1.6 | 26.6±1.0 |
| Z17 | 30.2±1.5 | 28.6±1.2 | 67.7±2.4 | C5 | 28.4±1.2 | 36.4±1.0 | 38.9±2.3 |
| Z18 | 22.9±2.1 | 32.9±2.5 | 38.3±2.0 | NNMb | 59.7±0.7 | 65.4±2.4 | 84.1±1.3 |
| Compd. | Curative activity/% | Protective activity/% | Inactivation activity/% | Compd. | Curative activity/% | Protective activity/% | Inactivation activity/% |
|---|---|---|---|---|---|---|---|
| Z1 | 31.5±4.6 | 17.9±3.4 | 37.5±0.8 | Z19 | 25.1±1.1 | 45.8±0.7 | 53.1±4.2 |
| Z2 | 32.3±2.7 | 56.3±3.0 | 32.3±2.7 | Z20 | 62.5±2.9 | 35.5±1.3 | 47.6±3.2 |
| Z3 | 44.8±0.4 | 45.8±2.5 | 44.8±0.4 | Z21 | 52.3±3.5 | 55.7±2.4 | 58.5±3.3 |
| Z4 | 51.2±2.2 | 35.8±2.5 | 23.0±1.9 | Z22 | 24.7±2.7 | 36.9±0.9 | 64.7±3.9 |
| Z5 | 66.9±3.2 | 67.4±1.1 | 39.8±0.2 | Z23 | 17.9±2.3 | 20.1±1.7 | 38.1±1.6 |
| Z6 | 34.7±2.1 | 44.1±3.4 | 33.6±0.8 | Z24 | 36.6±2.5 | 18.9±2.3 | 28.1±1.2 |
| Z7 | 26.2±2.1 | 71.1±0.9 | 26.2±2.1 | Z25 | 22.2±1.8 | 27.7±2.9 | 54.1±2.8 |
| Z8 | 34.6±2.6 | 55.9±3.1 | 46.8±1.8 | K1 | 25.5±1.1 | 32.1±2.0 | 33.7±1.4 |
| Z9 | 22.8±4.1 | 51.1±0.8 | 26.9±1.8 | K2 | 35.3±2.3 | 27.9±1.5 | 42.1±1.1 |
| Z10 | 45.6±3.7 | 63.0±1.9 | 45.6±3.7 | K3 | 38.7±1.4 | 35.6±2.0 | 23.3±0.9 |
| Z11 | 36.6±4.9 | 23.0±2.9 | 36.6±4.9 | K4 | 27.3±1.2 | 30.1±2.3 | 34.5±1.5 |
| Z12 | 66.6±2.9 | 70.4±2.7 | 69.5±1.3 | K5 | 40.2±2.3 | 34.4±1.7 | 37.5±1.2 |
| Z13 | 35.0±4.1 | 33.7±1.1 | 25.0±4.1 | C1 | 33.9±1.7 | 24.3±2.1 | 35.9±1.3 |
| Z14 | 32.3±1.2 | 34.6±2.2 | 61.5±1.3 | C2 | 29.9±2.0 | 35.8±1.6 | 27.9±2.1 |
| Z15 | 28.7±1.1 | 59.8±2.5 | 31.5±0.9 | C3 | 39.9±1.6 | 32.2±1.4 | 31.1±2.0 |
| Z16 | 19.8±0.8 | 38.9±1.0 | 63.4±2.9 | C4 | 43.3±1.9 | 33.2±1.6 | 26.6±1.0 |
| Z17 | 30.2±1.5 | 28.6±1.2 | 67.7±2.4 | C5 | 28.4±1.2 | 36.4±1.0 | 38.9±2.3 |
| Z18 | 22.9±2.1 | 32.9±2.5 | 38.3±2.0 | NNMb | 59.7±0.7 | 65.4±2.4 | 84.1±1.3 |
| Compd. | R1 | R2 | Regression equation | r | EC50/(μg•mL–1) | |
|---|---|---|---|---|---|---|
| Curative | Z5 | 4-Br | Br | y=0.9764x+2.7880 | 0.9798 | 184.3 |
| Z12 | 4-OCH3 | F | y=0.8768x+3.2688 | 0.9961 | 94.3 | |
| Z20 | 4-Br | OCH3 | y=0.9034x+2.9644 | 0.9973 | 179.2 | |
| NNMb | — | — | y=0.9338x+2.8199 | 0.9915 | 216.1 | |
| Protection | Z5 | 4-Br | Br | y=0.8797x+3.0150 | 0.9961 | 180.1 |
| Z7 | 4-OCH3 | CH3 | y=0.8934x+3.0322 | 0.9779 | 159.4 | |
| Z12 | 4-OCH3 | F | y=0.7942x+3.4156 | 0.9902 | 98.8 | |
| NNM | — | — | y=1.0142x+2.6898 | 0.9928 | 189.6 |
| Compd. | R1 | R2 | Regression equation | r | EC50/(μg•mL–1) | |
|---|---|---|---|---|---|---|
| Curative | Z5 | 4-Br | Br | y=0.9764x+2.7880 | 0.9798 | 184.3 |
| Z12 | 4-OCH3 | F | y=0.8768x+3.2688 | 0.9961 | 94.3 | |
| Z20 | 4-Br | OCH3 | y=0.9034x+2.9644 | 0.9973 | 179.2 | |
| NNMb | — | — | y=0.9338x+2.8199 | 0.9915 | 216.1 | |
| Protection | Z5 | 4-Br | Br | y=0.8797x+3.0150 | 0.9961 | 180.1 |
| Z7 | 4-OCH3 | CH3 | y=0.8934x+3.0322 | 0.9779 | 159.4 | |
| Z12 | 4-OCH3 | F | y=0.7942x+3.4156 | 0.9902 | 98.8 | |
| NNM | — | — | y=1.0142x+2.6898 | 0.9928 | 189.6 |
| Compd. | Xac | Psa | Xoo | |||||
|---|---|---|---|---|---|---|---|---|
| 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | |||
| Z1 | 25.1±1.1 | 21.2±1.2 | 35.4±2.4 | 22.7±2.2 | 25.4±2.4 | 12.7±0.5 | ||
| Z2 | 38.2±0.8 | 23.5±0.8 | 47.9±2.0 | 34.7±2.7 | 27.9±2.0 | 14.7±2.7 | ||
| Z3 | 23.9±5.4 | 18.4±0.9 | 52.9±4.8 | 44.9±2.3 | 32.9±4.8 | 10.9±2.3 | ||
| Z4 | 22.9±0.5 | 21.7±5.7 | 50.5±2.4 | 39.9±2.5 | 30.5±2.4 | 19.9±2.5 | ||
| Z5 | 43.5±2.7 | 31.3±1.7 | 25.4±5.4 | 12.7±1.2 | 45.4±1.4 | 22.7±1.2 | ||
| Z6 | 30.1±1.6 | 27.1±2.4 | 37.9±2.0 | 24.7±2.7 | 27.9±2.0 | 14.7±2.7 | ||
| Z7 | 24.5±1.2 | 12.4±8.2 | 61.8±5.7 | 44.5±3.6 | 11.8±2.7 | — | ||
| Z8 | 38.5±1.9 | 22.4±2.3 | 38.8±3.5 | 24.0±5.6 | 28.8±3.5 | 14.0±0.6 | ||
| Z9 | 36.7±1.8 | 33.6±0.8 | 28.0±2.6 | 11.2±3.0 | 28.0±2.6 | 11.2±3.0 | ||
| Z10 | 26.0±2.5 | 15.7±3.8 | 13.9±3.0 | 7.5±0.9 | 33.9±3.0 | 17.5±3.9 | ||
| Z11 | 37.7±1.8 | 26.4±1.8 | 27.3±1.1 | 11.2±4.3 | 27.3±3.1 | 11.2±4.3 | ||
| Z12 | 45.8±4.9 | 24.3±1.9 | 37.5±3.9 | 22.5±3.9 | 27.5±3.9 | 14.5±3.9 | ||
| Z13 | 57.8±2.1 | 35.7±2.1 | 27.4±4.3 | 15.1±4.6 | 27.4±4.3 | 15.1±2.1 | ||
| Z14 | 33.8±1.8 | 21.9±1.8 | 49.0±4.2 | 32.3±1.2 | 59.0±4.2 | 32.3±1.2 | ||
| Z15 | 32.1±1.6 | 21.5±1.6 | 27.2±4.6 | 15.1±1.2 | 27.2±4.6 | 16.1±1.6 | ||
| Z16 | 42.6±3.3 | 21.2±3.5 | 15.6±4.2 | 9.1±0.2 | 35.6±4.2 | 23.1±1.2 | ||
| Z17 | 35.6±1.8 | 23.1±2.2 | 36.5±1.0 | 25.2±2.1 | 61.5±1.0 | 45.2±2.1 | ||
| Z18 | 33.1±3.8 | 20.7±0.3 | 35.4±3.3 | 28.8±4.3 | 25.4±3.3 | 13.8±4.3 | ||
| Z19 | 35.7±2.7 | 15.5±2.5 | 29.2±4.6 | 13.2±2.6 | 29.2±4.6 | 12.2±4.6 | ||
| Z20 | 59.7±2.6 | 27.3±0.5 | 32.2±0.8 | 21.6±0.6 | 22.2±0.8 | 11.6±3.6 | ||
| Z21 | 21.2±.5 | 11.1±2.2 | 25.2±0.6 | 11.5±1.6 | 35.2±0.6 | 17.5±1.6 | ||
| Z22 | 23.3±1.4 | 15.6±1.3 | 35.8±0.1 | 21.6±2.1 | 45.8±0.1 | 21.6±3.1 | ||
| Z23 | 38.9±2.6 | 27.1±1.5 | 42.1±1.2 | 31.0±1.0 | 32.1±3.2 | 18.0±2.0 | ||
| Z24 | 32.2±3.3 | 16.7±0.6 | 36.1±2.3 | 29.3±5.4 | 26.1±2.3 | 19.3±1.4 | ||
| Z25 | 57.5±2.7 | 45.4±0.3 | 37.2±0.9 | 27.5±3.2 | 67.2±0.9 | 57.2±3.2 | ||
| TCb | 52.2±0.5 | 46.1±1.8 | 52.1±3.4 | 42.2±3.9 | 55.4±3.4 | 42.7±1.2 | ||
| BTb | 47.7±1.0 | 33.0±2.6 | 49.2±3.0 | 36.3±1.8 | 50.9±2.0 | 36.9±2.7 | ||
| Compd. | Xac | Psa | Xoo | |||||
|---|---|---|---|---|---|---|---|---|
| 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | |||
| Z1 | 25.1±1.1 | 21.2±1.2 | 35.4±2.4 | 22.7±2.2 | 25.4±2.4 | 12.7±0.5 | ||
| Z2 | 38.2±0.8 | 23.5±0.8 | 47.9±2.0 | 34.7±2.7 | 27.9±2.0 | 14.7±2.7 | ||
| Z3 | 23.9±5.4 | 18.4±0.9 | 52.9±4.8 | 44.9±2.3 | 32.9±4.8 | 10.9±2.3 | ||
| Z4 | 22.9±0.5 | 21.7±5.7 | 50.5±2.4 | 39.9±2.5 | 30.5±2.4 | 19.9±2.5 | ||
| Z5 | 43.5±2.7 | 31.3±1.7 | 25.4±5.4 | 12.7±1.2 | 45.4±1.4 | 22.7±1.2 | ||
| Z6 | 30.1±1.6 | 27.1±2.4 | 37.9±2.0 | 24.7±2.7 | 27.9±2.0 | 14.7±2.7 | ||
| Z7 | 24.5±1.2 | 12.4±8.2 | 61.8±5.7 | 44.5±3.6 | 11.8±2.7 | — | ||
| Z8 | 38.5±1.9 | 22.4±2.3 | 38.8±3.5 | 24.0±5.6 | 28.8±3.5 | 14.0±0.6 | ||
| Z9 | 36.7±1.8 | 33.6±0.8 | 28.0±2.6 | 11.2±3.0 | 28.0±2.6 | 11.2±3.0 | ||
| Z10 | 26.0±2.5 | 15.7±3.8 | 13.9±3.0 | 7.5±0.9 | 33.9±3.0 | 17.5±3.9 | ||
| Z11 | 37.7±1.8 | 26.4±1.8 | 27.3±1.1 | 11.2±4.3 | 27.3±3.1 | 11.2±4.3 | ||
| Z12 | 45.8±4.9 | 24.3±1.9 | 37.5±3.9 | 22.5±3.9 | 27.5±3.9 | 14.5±3.9 | ||
| Z13 | 57.8±2.1 | 35.7±2.1 | 27.4±4.3 | 15.1±4.6 | 27.4±4.3 | 15.1±2.1 | ||
| Z14 | 33.8±1.8 | 21.9±1.8 | 49.0±4.2 | 32.3±1.2 | 59.0±4.2 | 32.3±1.2 | ||
| Z15 | 32.1±1.6 | 21.5±1.6 | 27.2±4.6 | 15.1±1.2 | 27.2±4.6 | 16.1±1.6 | ||
| Z16 | 42.6±3.3 | 21.2±3.5 | 15.6±4.2 | 9.1±0.2 | 35.6±4.2 | 23.1±1.2 | ||
| Z17 | 35.6±1.8 | 23.1±2.2 | 36.5±1.0 | 25.2±2.1 | 61.5±1.0 | 45.2±2.1 | ||
| Z18 | 33.1±3.8 | 20.7±0.3 | 35.4±3.3 | 28.8±4.3 | 25.4±3.3 | 13.8±4.3 | ||
| Z19 | 35.7±2.7 | 15.5±2.5 | 29.2±4.6 | 13.2±2.6 | 29.2±4.6 | 12.2±4.6 | ||
| Z20 | 59.7±2.6 | 27.3±0.5 | 32.2±0.8 | 21.6±0.6 | 22.2±0.8 | 11.6±3.6 | ||
| Z21 | 21.2±.5 | 11.1±2.2 | 25.2±0.6 | 11.5±1.6 | 35.2±0.6 | 17.5±1.6 | ||
| Z22 | 23.3±1.4 | 15.6±1.3 | 35.8±0.1 | 21.6±2.1 | 45.8±0.1 | 21.6±3.1 | ||
| Z23 | 38.9±2.6 | 27.1±1.5 | 42.1±1.2 | 31.0±1.0 | 32.1±3.2 | 18.0±2.0 | ||
| Z24 | 32.2±3.3 | 16.7±0.6 | 36.1±2.3 | 29.3±5.4 | 26.1±2.3 | 19.3±1.4 | ||
| Z25 | 57.5±2.7 | 45.4±0.3 | 37.2±0.9 | 27.5±3.2 | 67.2±0.9 | 57.2±3.2 | ||
| TCb | 52.2±0.5 | 46.1±1.8 | 52.1±3.4 | 42.2±3.9 | 55.4±3.4 | 42.7±1.2 | ||
| BTb | 47.7±1.0 | 33.0±2.6 | 49.2±3.0 | 36.3±1.8 | 50.9±2.0 | 36.9±2.7 | ||
| Bacteria | Compd. | R1 | R2 | Toxic regression equation | r | EC50/(μg•mL–1) |
|---|---|---|---|---|---|---|
| Xac | Z13 | H | F | y=0.8814x+3.3228 | 0.9589 | 79.9 |
| Z20 | 4-Br | OCH3 | y=0.9858x+3.2922 | 0.9858 | 60.6 | |
| Z25 | 4-Br | NO2 | y=0.7713x+3.0270 | 0.9723 | 23.5 | |
| TC | y=0.8186x+3.4996 | 0.9598 | 68.1 | |||
| BT | y=0.7975x+3.4148 | 0.9890 | 97.2 | |||
| Psa | Z7 | 4-OCH3 | CH3 | y=1.0266x+3.2172 | 0.9901 | 63.2 |
| TC | y=0.9032x+3.2888 | 0.9949 | 78.5 | |||
| BT | y=0.7909x+3.4402 | 0.9969 | 93.8 | |||
| Xoo | Z17 | 4-OCH3 | OCH3 | y=1.2518x+2.7652 | 0.9790 | 60.9 |
| Z25 | 4-Br | NO2 | y=0.8836x+3.8606 | 0.9909 | 19.5 | |
| TCb | y=0.7594x+3.5671 | 0.9856 | 77.1 | |||
| BTb | y=0.6777x+3.6569 | 0.9818 | 95.9 |
| Bacteria | Compd. | R1 | R2 | Toxic regression equation | r | EC50/(μg•mL–1) |
|---|---|---|---|---|---|---|
| Xac | Z13 | H | F | y=0.8814x+3.3228 | 0.9589 | 79.9 |
| Z20 | 4-Br | OCH3 | y=0.9858x+3.2922 | 0.9858 | 60.6 | |
| Z25 | 4-Br | NO2 | y=0.7713x+3.0270 | 0.9723 | 23.5 | |
| TC | y=0.8186x+3.4996 | 0.9598 | 68.1 | |||
| BT | y=0.7975x+3.4148 | 0.9890 | 97.2 | |||
| Psa | Z7 | 4-OCH3 | CH3 | y=1.0266x+3.2172 | 0.9901 | 63.2 |
| TC | y=0.9032x+3.2888 | 0.9949 | 78.5 | |||
| BT | y=0.7909x+3.4402 | 0.9969 | 93.8 | |||
| Xoo | Z17 | 4-OCH3 | OCH3 | y=1.2518x+2.7652 | 0.9790 | 60.9 |
| Z25 | 4-Br | NO2 | y=0.8836x+3.8606 | 0.9909 | 19.5 | |
| TCb | y=0.7594x+3.5671 | 0.9856 | 77.1 | |||
| BTb | y=0.6777x+3.6569 | 0.9818 | 95.9 |

| [1] |
Silva R. N.; Monteiro V. N.; Steindorff A. S.; Gomes E. V.; Noronha E. F.; Ulhoa C. J. Fungal Biol. 2019, 123, 565.
doi: 10.1016/j.funbio.2019.06.010 |
| [2] |
Tang X.; Zhang C.; Chen M.; Xue Y. N.; Liu T. T.; Xue W. New J. Chem. 2020, 44, 2374.
doi: 10.1039/C9NJ05867B |
| [3] |
Wei C. L.; Zhao L.; Sun Z. R.; Hu D. Y.; Song B. A. Pestic. Biochem. Physiol. 2020, 166, 104568.
doi: 10.1016/j.pestbp.2020.104568 |
| [4] |
Pan X. Y.; Xu S.; Wu J.; Luo J. Y.; Duan Y. B.; Wang J. X.; Zhang F.; Zhou M. G. Pestic. Biochem. Physiol. 2018, 145, 8.
doi: 10.1016/j.pestbp.2017.12.003 |
| [5] |
Shi J.; Ding M. H.; Luo N.; Wan S. R.; Li P. J.; Li J. H.; Bao X. P. J. Agric. Food Chem. 2020, 68, 9613.
doi: 10.1021/acs.jafc.0c01365 |
| [6] |
Mansfield J.; Genin S.; Magori S.; Citovsky V.; Sriariyanum M.; Ronald P.; Dow M.; Verdier V.; Beer S. V.; Machado M. A.; Salmond I.; Toth G.; Foster G. D. Mol. Plant Pathol. 2012, 13, 614.
doi: 10.1111/j.1364-3703.2012.00804.x pmid: 22672649 |
| [7] |
Monchiero M.; Gullino M.; Lodovica; Pugliese, M.; Spadaro, D.; Garibaldi, A. Australas. Plant Pathol. 2015, 44, 13.
doi: 10.1007/s13313-014-0328-1 |
| [8] |
Graham J. H.; Gottwald T. R.; Cubero J.; Achor D. S. Mol. Plant Pathol. 2004, 5, 1.
doi: 10.1046/j.1364-3703.2004.00197.x pmid: 20565577 |
| [9] |
Peng J. W.; Yin X. D.; Li H.; Ma K. Y.; Zhang Z. J.; Zhou R.; Wang Y. L.; Hu G. F.; Liu Y. Q. J. Agric. Food Chem. 2021, 69, 4604.
doi: 10.1021/acs.jafc.0c05475 |
| [10] |
Liu T. T.; Peng F.; Zhu Y. Y.; Cao X.; Wang Q. F.; Liu F.; Liu L. W.; Xue W. Arabian J. Chem. 2022, 15, 104019.
doi: 10.1016/j.arabjc.2022.104019 |
| [11] |
Zhan X. P.; Xu Y.; Qi Q.; Wang Y. L.; Shi H. Y.; Mao Z. M. Chem. Biodiversity 2018, 15, e1700513.
doi: 10.1002/cbdv.v15.3 |
| [12] |
El-Sayed A. A.; Ismail M. F.; Amr A. E. E.; Naglah A. M. Molecules 2019, 24, 3787.
doi: 10.3390/molecules24203787 |
| [13] |
Zhang L.; Chen Q.; Li X. Q.; Wu S. Q.; Wan J. L.; Ouyang G. P. J. Heterocycl. Chem. 2018, 55, 743.
doi: 10.1002/jhet.v55.3 |
| [14] |
El-Bordany E. A.; Ali R. S.; J. Heterocycl. Chem. 2018, 55, 1223.
doi: 10.1002/jhet.v55.5 |
| [15] |
Wang X.; Li P.; Li Z. N.; Yin J.; He M.; Xue W.; Chen Z. W.; Song B. A. J. Agric. Food Chem. 2013, 61, 9575.
doi: 10.1021/jf403193q |
| [16] |
Ran L. L.; Yang H. Y.; Luo L. Z.; Huang M. X.; Hu D. Y. J. Agric. Food Chem. 2020, 68, 5302.
doi: 10.1021/acs.jafc.0c00686 |
| [17] |
Tomar V.; Bhattacharjee G.; Kamaluddin C.; Rajakumar S.; Srivastava K.; Puri S. K. Eur. J. Med. Chem. 2010, 45, 745.
doi: 10.1016/j.ejmech.2009.11.022 pmid: 20022412 |
| [18] |
Tan B. C.; Tan S. K.; Ming W. S.; Wong S. M.; Nabeel A.; Abd R. N.; Norzulaani K. M. Evid-Based Compl. Alt. 2015, 2015, 451870.
|
| [19] |
Coskun D.; Erkisa M.; Ulukaya E.; Coskun M. F.; Ari F. Eur. J. Med. Chem. 2017, 136, 212.
doi: S0223-5234(17)30378-1 pmid: 28494257 |
| [20] |
Li J. F.; Li D.; Xu Y. M.; Guo Z. B.; Liu X.; Yang H.; Wu L. C.; Wang L. S. Bioorg. Med. Chem. Lett. 2017, 27, 602.
doi: 10.1016/j.bmcl.2016.12.008 |
| [21] |
Gan X. H.; Hu D. Y.; Wang Y. J.; Yu L.; Song B. A. J. Agric. Food Chem. 2017, 65, 4367.
doi: 10.1021/acs.jafc.7b00958 |
| [22] |
Yan Y. K.; Xu Q.; Gao Y.; Liu H.; Tang X. R. Chin. J. Org. Chem. 2018, 38, 1763. (in Chinese)
doi: 10.6023/cjoc201712027 |
|
(严映坤, 徐侨, 高扬, 刘辉, 唐孝荣, 有机化学, 2018, 38, 1763.)
doi: 10.6023/cjoc201712027 |
|
| [23] |
Wang H. X.; Liu H. Y.; Li W.; Zhang S.; Wu Z.; Li X.; Li C. W.; Liu Y. M.; Chen B. Q. Med. Chem. Res. 2019, 28, 203.
doi: 10.1007/s00044-018-2276-8 |
| [24] |
Guo T.; Xia R. J.; Liu T. T.; Peng F.; Tang X. M.; Zhou Q.; Luo H.; Xue W. Chem. Biodiversity 2020, 17, e2000025.
doi: 10.1002/cbdv.v17.4 |
| [25] |
Tang X.; Su S. J.; Chen M.; He J.; Xia R. J.; Guo T.; Chen Y.; Zhang C.; Wang J.; Xue W. RSC Adv. 2019, 9, 6011.
doi: 10.1039/c9ra00618d |
| [26] |
He J.; Tang X. M.; Zhou Q.; Peng F.; Liu T. T.; Liu L. W.; He M.; Xie C. W.; Xue W. Chin. J. Org. Chem. 2021, 41, 717. (in Chinese)
|
|
(贺军, 唐雪梅, 周清, 彭峰, 刘婷婷, 柳立伟, 贺鸣, 谢承卫, 薛伟, 有机化学, 2021, 41, 717.)
|
|
| [27] |
Chen Y.; Li P.; Chen M.; Su S. J.; He J.; Zhang M.; Liu L. W.; Xue W. Chin. J. Org. Chem. 2022, 42, 2813. (in Chinese)
|
|
(陈英, 李普, 陈梅, 苏时军, 贺军, 张敏, 柳立伟, 薛伟, 有机化学, 2019, 39, 2813.)
doi: 10.6023/cjoc201903048 |
|
| [28] |
Wang L. L.; Li C.; Zhang Y. Y.; Qiao C. H.; Ye Y. H. J. Agric. Food. Chem. 2013, 61, 8632.
doi: 10.1021/jf402388x |
| [29] |
Saroj A.; Pragadheesh V. S.; Palanivelu P.; Yadav A.; Singh S. C.; Samad A.; Negi A. S.; Chanotiya C. S. Ind. Crops Prod. 2015, 74, 327.
doi: 10.1016/j.indcrop.2015.04.065 |
| [30] |
Jiang D. H.; Chen J. X.; Zan N. N.; Li C. Y.; Hu D. Y.; Song B. A. J. Agric. Food Chem. 2021, 69, 12126.
doi: 10.1021/acs.jafc.1c02467 |
| [31] |
Guo S. X.; Zhao W.; Wang Y. Y.; Zhang W.; Chen S. H.; Wei P. P.; Wu J. J. Agric. Food Chem. 2021, 69, 12891.
doi: 10.1021/acs.jafc.1c03586 |
| [32] |
Yuan T.; Wang Z. X.; Liu D.; Zeng H. N.; Liang J. C.; Hu D. Y.; Gan X. H. Pest Manage. Sci. 2022, 78, 1749.
doi: 10.1002/ps.v78.4 |
| [33] |
Peng F.; Liu T.T.; Zhu Y. Y.; Liu F.; Cao X.; Wang Q. F.; Liu L. W.; Xue W. Pest Manage. Sci. 2022, 79, 274.
doi: 10.1002/ps.v79.1 |
| [34] |
Zhang C.; Jiang S. C.; Chen Y.; Guo T.; Xia R. J.; Tang X.; Chen L. J.; He M.; Xue W. Chin. J. Org. Chem. 2019, 39, 1160. (in Chinese)
doi: 10.6023/cjoc201809040 |
|
(张橙, 蒋仕春, 陈英, 郭涛, 夏榕娇, 陈丽娟, 贺鸣, 薛伟, 有机化学, 2019, 39, 1160.)
doi: 10.6023/cjoc201809040 |
| [1] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [2] | 霍海波, 李桂霞, 王世军, 韩春, 师宝君, 李健. 新型γ-咔啉衍生物的合成及其抑菌活性研究[J]. 有机化学, 2024, 44(1): 204-215. |
| [3] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [4] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [5] | 徐光利, 许静, 徐海东, 崔香, 舒兴中. 过渡金属催化烯烃和炔烃合成1,3-共轭二烯化合物研究进展[J]. 有机化学, 2023, 43(6): 1899-1933. |
| [6] | 白林盛, 洪鹏, 应安国. 功能化聚丙烯腈纤维促进有机反应的研究进展[J]. 有机化学, 2023, 43(4): 1241-1270. |
| [7] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [8] | 窦谦, 汪太民, 房丽晶, 翟宏斌, 程斌. 光诱导铁催化在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1386-1415. |
| [9] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [10] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [11] | 马彪, 章淼淼, 李占宇, 彭进松, 陈春霞. 无过渡金属催化的Suzuki-Type交叉偶联反应研究进展[J]. 有机化学, 2023, 43(2): 455-470. |
| [12] | 唐朵朵, 黄丹凤, 王克虎, 马虎, 冯杨, 任园园, 王君姣, 胡雨来. 锡粉促进下1,3-二取代-1,3-二氢异苯并呋喃化合物的合成[J]. 有机化学, 2023, 43(12): 4227-4238. |
| [13] | 陈泗林, 杨芸辉, 陈超, 王从洋. 过渡金属催化的酮羰基导向C—H键官能化反应进展[J]. 有机化学, 2023, 43(1): 1-16. |
| [14] | 刘威琴, 邵利辉, 李成朋, 邹雅玉, 龙海洮, 李焱, 戈强胜, 王贞超, 欧阳贵平. 3-腙喹唑啉酮衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(1): 214-222. |
| [15] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||