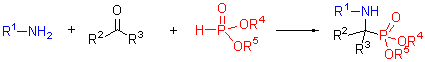
| [1] (a) Kabachnik, M. I.; Medved, T. Y. Dokl. Akad. Nauk SSSR 1952, 83, 689 [Chem. Abstr. 1953, 47, 2724]. (b) Kabachnik, M. I.; Medved, T. Y. Izv. Akad. Nauk SSSR, Ser. Khim. 1953, 1126. (c) Kabachnik, M. I.; Medved, T. Y. Izv. Akad. Nauk SSSR, Ser. Khim. 1954, 1024 (d) Fields, E. J. Am. Chem. Soc. 1952, 74, 1528.[2] Giannousis, P. P.; Bartlett, P. A. J. Med. Chem. 1987, 30, 1603.[3] Smith, A. B.; Taylor, C. M.; Benkovic, S. J.; Hirschman, R. Tetrahedron Lett. 1994, 35, 6853.[4] Barder, A. Aldrichim. Acta 1988, 21, 15.[5] Atherton, F. R.; Hassal, C. H.; Lambert, R. W. J. Med. Chem. 1986, 29, 29.[6] Baylis, E. K.; Campbell, C. D.; Dingwall, J. G. J. Chem. Soc. Perkin Trans. 1 1984, 845.[7] Alonso, E.; Solis, A.; Pozo, C. Synlett 2000, 698.[8] Allen, M. C.; Fuhrer, W.; Tuck, B. J. Med. Chem. 1989, 32, 1652.[9] Bird, J.; Mello, R. C. D.; Harper, G. P. J. Med. Chem. 1994, 37, 158.[10] Ding, J.; Fraser, M. E.; Meyer, J. H. J. Am. Chem. Soc. 1998, 120, 4610.[11] Smith, W. W.; Bartlett, P. A. J. Am. Chem. Soc. 1998, 120, 4622.[12] Maier, L.; Diel, P. J. Phosphorous, Sulfur Silicon Relat. Elem. 1991, 57, 57.[13] Kukhar, V. P.; Hudson, H. R. Aminophosphonic and Aminophosphinic Acids Chemistry and Biological Activity, John Wiley & Sons Ltd, Chichester, 2000.[14] Dhawan, B.; Redmore, D. Phosphorous, Sulfur Silicon Relat. Elem. 1987, 32, 119.[15] Song, B. A.; Wu, Y. L.; He, X. Q. Chin. J. Org. Chem. 2003, 23, 933 (in Chinese). (宋宝安, 吴杨兰, 何湘琼等, 有机化学, 2003, 23, 933.)[16] Ma, J. A. Chem. Soc. Rev. 2006, 35, 630.[17] Zhang, J. F.; Cui, Z. W.; Miao, Z. W.; Chen, R. Y. Chin. J. Org.[18] Ranu, B. C.; Hajra, A.; Jana, U. Org. Lett. 1999, 1, 1141.[19] Xu, F.; Luo, Y. Q.; Deng, M. Y.; Shen, Q. Eur. J. Org.Chem. 2003, 4728.[20] Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2007, 72, 3158.[21] Kaboudin, B.; Nazari, R. Tetrahedron Lett. 2001, 42, 8211.[22] Heydari, A.; Khaksar, S.; Tajbakhsh, M. Tetrahedron Lett. 2009, 50, 77.[23] Saito, B.; Egami, H.; Katsuki, T. J. Am. Chem. Soc. 2007, 129, 1978.[24] (a) Sasai, H.; Arai, S.; Tahara, Y.; Shibasaki, M. J. Org. Chem. 1995, 60, 6656. (b) GrJger, H.; Saida,Y.; Sasai, H.; Yamaguchi, K.; Martens, J.; Shibasaki, M. J. Am. Chem. Soc. 1998, 120, 3089. (c) Kobayashi, S.; Kiyohara, H.; Nakamura,Y.; Matsubara, R. J. J. Am. Chem. Soc. 2004, 126, 6558.[25] Joly, G. D.; Jacobsen, E. N. J. Am. Chem. Soc. 2004, 126, 4102.[26] Akiyama, T. H.; Morita, H.; Itoh, J.; Fuchibe, K. Org. Lett. 2005, 7, 2583.[27] Xu, C.; Goddard, R.; Buth, G.; List, B. Angew. Chem., Int. Ed. Engl. 2008, 47, 5079.[28] Wang, L.; Cui, S.; Meng, W.; Zhang, G.; Nie, J.; Ma, J. Chin. Sci. Bull. 2010, 55, 1729.[29] Zhou, X.; Shang, D.; Zhang, Q.; Lin, L.; Liu, X.; Feng, X. Org. Lett. 2009, 11, 1401.[30] Wang, Y.; Wang, F.;Wang, Y.; Miao, Z.; Chen, R. Adv. Synth. Catal. 2008, 350, 2339.[31] He, F.; Meng, F.; Song, X.; Hu,W; Xu, J. Org. Lett. 2009, 17, 3922.[32] Meng, F.; Xu, J. Amino Acids 2010, 39, 533.[33] Tibhe, G. D.; Selene, L. R.; Elena, V. D.; Oscar, G. B.; Mario, O. Eur. J. Org. Chem. 2010, 6573.[34] Cui, Z.; Zhang, J.; Wang, F.; Wang, Y.; Miao, Z.; Chen, R. Carbohydr. Res. 2008, 343, 2530.[35] Xu, J.-X.; Gao, Y.-H. Synthesis 2006, 783.[36] Liu, H.; Cai, S. Z.; Xu, J. X. J. Peptide Sci. 2006, 12, 337. |