有机化学 ›› 2019, Vol. 39 ›› Issue (10): 2802-2807.DOI: 10.6023/cjoc201904057 上一篇 下一篇
研究论文
收稿日期:2019-04-24
修回日期:2019-06-12
发布日期:2019-07-09
通讯作者:
王薪,孙凯
E-mail:wangx933@nenu.edu.cn;sunk468@nenu.edu.cn
基金资助:
Wang Xin*( ), Mu Shiqiang, Sun Ting, Sun Kai*(
), Mu Shiqiang, Sun Ting, Sun Kai*( )
)
Received:2019-04-24
Revised:2019-06-12
Published:2019-07-09
Contact:
Wang Xin,Sun Kai
E-mail:wangx933@nenu.edu.cn;sunk468@nenu.edu.cn
Supported by:文章分享
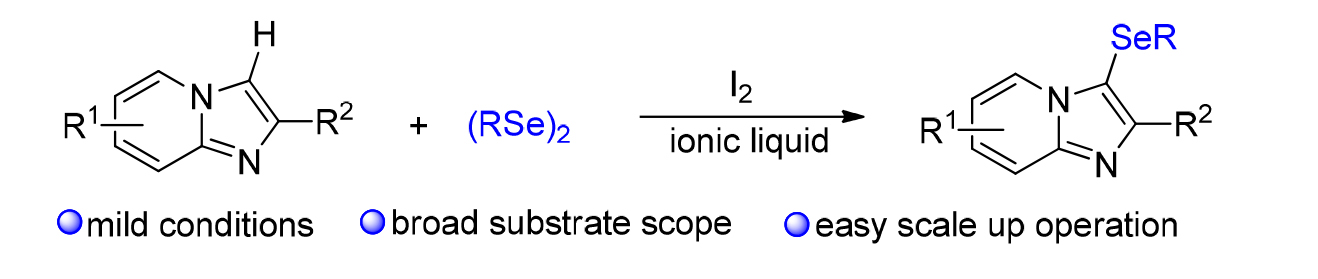
发展了一种环境相对友好的、咪唑并吡啶衍生物和有机硒化合物C-3位的硒化反应, 目标产物能以中等到优的收率获得. 初步机理研究表明, 该硒化反应经历了亲电加成反应机制, 具有反应条件温和、底物范围宽泛、易于放大量生产等特点. 因此, 该策略在合成含氮和含硒分子中具有重要的应用前景.
王薪, 穆石强, 孙婷, 孙凯. 咪唑并吡啶在离子液中环境友好型的C-3位硒化反应[J]. 有机化学, 2019, 39(10): 2802-2807.
Wang Xin, Mu Shiqiang, Sun Ting, Sun Kai. Eco-friendly C-3 Selenation of Imidazo[1,2-a]pyridines in Ionic Liquid[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2802-2807.
| Entry | Iodine source | Oxidant | Solvent | Yieldb/% of 3a/4 |
|---|---|---|---|---|
| 1 | KI | TBHP | EtOAc | 41/0c |
| 2 | KI | TBHP | EtOH | 23/0c |
| 3 | KI | TBHP | H2O | 0/0 c |
| 4 | KI | TBHP | DMSO | 55/0c |
| 5 | KI | TBHP | Ionic liquid I | 67/0d |
| 6 | KI | TBHP | Ionic liquid II | 43/0e |
| 7 | I2 | TBHP | Ionic liquid I | 51/Tracef |
| 8 | I2 | No | Ionic liquid I | 84/Traceg |
| 9 | I2 | No | Ionic liquid I | 33/23h |
| 10 | I2 | No | Ionic liquid I | 56/Tracei |
| Entry | Iodine source | Oxidant | Solvent | Yieldb/% of 3a/4 |
|---|---|---|---|---|
| 1 | KI | TBHP | EtOAc | 41/0c |
| 2 | KI | TBHP | EtOH | 23/0c |
| 3 | KI | TBHP | H2O | 0/0 c |
| 4 | KI | TBHP | DMSO | 55/0c |
| 5 | KI | TBHP | Ionic liquid I | 67/0d |
| 6 | KI | TBHP | Ionic liquid II | 43/0e |
| 7 | I2 | TBHP | Ionic liquid I | 51/Tracef |
| 8 | I2 | No | Ionic liquid I | 84/Traceg |
| 9 | I2 | No | Ionic liquid I | 33/23h |
| 10 | I2 | No | Ionic liquid I | 56/Tracei |
| [1] |
(a) Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888
doi: 10.2174/138955707781662645 |
|
(b) Baviskar, A. T.; Amrutkar, S. M.; Trivedi, N.; Chaudhary, V.; Nayak, A.; Guchhait, S. K.; Banerjee, U. C.; Bharatam, P. V.; Kundu, C.N. ACS Med. Chem. Lett. 2015, 6, 481.
doi: 10.2174/138955707781662645 |
|
| [2] |
(a) Meng, T.; Wang, W.; Zhang, Z.; Ma, L.; Zhang, Y.; Miao, Z.; Shen, J. Bioorg. Med. Chem. 2014, 22, 848.
doi: 10.1016/j.bmc.2013.12.004 |
|
(b) Gallud, A.; Vaillantm, O.; Maillard, L. T.; Arama, D. P.; Dubois, J.; Maynadier, M.; Lisowski, V.; Garcia, M.; Martinez, J.; Masurier, N. Eur. J. Med. Chem. 2014, 75, 382.
doi: 10.1016/j.bmc.2013.12.004 |
|
|
(c) Zhao, Y.-X.; Ding, Y.-Y.; Lü, Y.-T.; Kang, C.-M. Chin. J. Org. Chem. 2019, 39, 1304.(in Chinese).
doi: 10.1016/j.bmc.2013.12.004 |
|
|
( 赵鑫雨, 丁洋洋, 吕英涛, 康从民, 有机化学, 2019, 39, 1304.)
doi: 10.1016/j.bmc.2013.12.004 |
|
| [3] |
For selected papers, see: (a) Toure, B. B.; Lane, B. S.; Sames, D. Org. Lett. 2006, 8, 1979.
doi: 10.1021/ol053021c |
|
(b) Fu, H.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473.
doi: 10.1021/ol053021c |
|
|
(c) Choy, P. Y.; Luk, K. C.; Wu, Y.; So, C. M.; Wang, L.; Kwong, F.Y. J. Org. Chem. 2015, 80, 1457.
doi: 10.1021/ol053021c |
|
| [4] | For selected papers, see: (a) Koubachi, J.; Kazzouli, S. E.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumeta, G. Synthesis 2008,2537. |
| (b) Zhan, H.; Zhao, L.; Li, N.; Chen, L.; Liu, J.; Liao, J.; Cao, H. RSC Adv. 2014, 4, 32013. | |
| (c) Ghosh, M.; Naskar, A.; Mitra, S.; Hajra, A. Eur. J. Org. Chem. 2015, 2015, 715. | |
| [5] |
Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80, 1332.
doi: 10.1021/jo502928e |
| [6] |
(a) Lei, S.; Chen, G.; Mai, Y.; Chen, L.; Cai, H.; Tan, J.; Cao, H. Adv. Synth. Catal. 2016, 358, 67.
doi: 10.1002/adsc.v358.1 |
|
(b) Gao, Y.; Lu, W.; Liu, P.; Sun, P. J. Org. Chem. 2016, 81, 2482.
doi: 10.1002/adsc.v358.1 |
|
| [7] |
Liu, P.; Gao, Y.; Gu, W.; Shen, Z.; Sun, P. J. Org. Chem. 2015, 80, 11559.
doi: 10.1021/acs.joc.5b01961 |
| [8] |
For selected papers see: (a) Ravi, C.; Mohan, D. C.; Adimurthy, S. . Org. Lett 2014, 16, 2978.
doi: 10.1021/ol501117z |
|
(b) Gao, Z.; Zhu, X.; Zhang, R. RSC. Adv. 2014, 4, 19891.
doi: 10.1021/ol501117z |
|
|
(c) Bagdi, A. K.; Mitra, S.; Ghosh, M.; Hajra, A. Org. Biomol. Chem. 2015, 13, 3314.
doi: 10.1021/ol501117z |
|
|
(d) Rafique, J.; Saba, Sumbal.; Rosário, A. R.; Braga, A.L. Chem. Eur. J. 2016, 22, 11854.
doi: 10.1021/ol501117z |
|
|
(e) Rafique, J.; Saba, S.; Franco, M. S.; Bettanin, L.; Schneider, A. R.; Silva, L. T.; Braga, A.L. Chem. Eur. J. 2018, 16, 880.
doi: 10.1021/ol501117z |
|
|
(f) Xie, L.-Y.; Peng, S.; Fan, T.-G.; Liu, Y.-F.; Sun, M.; Jiang, L.-L.; Wang, X.-X.; Cao, Z.; He, W.-M. Sci. China Chem. 2019, 62, 460.
doi: 10.1021/ol501117z |
|
| [9] |
For selected papers see: (a) Toure, B. B.; Lane, B. S.; Sames, D. Org. Lett. 2006, 8, 1979.
doi: 10.1021/ol053021c |
|
(b) Cao, H.; Zhan, H.; Lin, Y.; Lin, X.; Du, Z.; Jiang, H. Org. Lett. 2012, 14, 1688.
doi: 10.1021/ol053021c |
|
|
(c) Fu, H.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473.
doi: 10.1021/ol053021c |
|
| [10] |
For selected papers see : (a) Li, Z.; Hong, J.; Zhou, X. Tetrahedron 2011, 67, 3690.
doi: 10.1016/j.tet.2011.03.067 |
|
(b) Ravi, C; Mohan, D. C.; Adimurthy, S. Org. Lett. 2014, 16, 2978.
doi: 10.1016/j.tet.2011.03.067 |
|
|
(c) Liu, S.; Xi, H.; Zhang, J.; Wu, X.; Gao, Q.; Wu, A. Org. Biomol. Chem. 2015, 13, 8807.
doi: 10.1016/j.tet.2011.03.067 |
|
|
(d) Huang, X.; Wang, S.; Li, B.; Wang, X.; Ge, Z.; Li, R. RSC Adv. 2015, 5, 22654.
doi: 10.1016/j.tet.2011.03.067 |
|
| [11] |
For selected papers see: (a) Cao, H.; Lei, S.; Li, N.; Chen, L.; Liu, J.; Cai, H.; Qiu, S.; Tan, J. Chem. Commun. 2015, 51, 1823.
doi: 10.1039/C4CC09134E |
|
(b) Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80, 1332.
doi: 10.1039/C4CC09134E |
|
|
(c) Mitra, S.; Ghosh, M.; Mishra, S.; Hajra, A. J. Org. Chem. 2015, 80, 8275.
doi: 10.1039/C4CC09134E |
|
|
(d) Yang, D.; Yan, K.; Wei, W.; Li, G.; Lu, S.; Zhao, C.; Tian, L.; Wang, H. J. Org. Chem. 2015, 80, 11073.
doi: 10.1039/C4CC09134E |
|
|
(e) Sun, K.; Li, S.-J.; Chen, X.-L.; Liu, Y.; Huang, X.-Q.; Wei, D.-H.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Chem. Commun. 2019, 55, 2861.
doi: 10.1039/C4CC09134E |
|
| [12] |
For selected works see: (a) Mugesh, G.; du Mont, W. W.; Sies, H. Chem. Rev. 2001, 101, 2125.
doi: 10.1021/cr000426w |
|
(b) Mugesh, G.; Singh, H.B. Acc. Chem. Res. 2002, 35, 226.
doi: 10.1021/cr000426w |
|
|
(c) Nogueira, C. W.; Zeni, G.; Rocha, JB. T.. Chem. Rev. 2004, 104, 6255.
doi: 10.1021/cr000426w |
|
|
(d) Rhoden, C. R. B.; Zeni, G. Org. Biomol. Chem. 2011, 9, 1301.
doi: 10.1021/cr000426w |
|
|
(e) Liu, M.-X.; Li, Y.-M.; Yu, L.; Xu, Q.; Jiang, X.-F. Sci. China Chem. 2018, 6, 294.
doi: 10.1021/cr000426w |
|
|
(f) Lu, L.-H.; Zhou, S.-J.; He, W.-B.; Xia, W.; Chen, P.; Yu, X.; Xu, X.; He, W.-M. Org. Biomol. Chem. 2018, 16, 9064.
doi: 10.1021/cr000426w |
|
|
(g) Wu, C.; Xiao, H.-J.; Wang, S.-W.; Tang, M.-S.; Tang, Z.-L.; Xia, W.; Li, W.-F.; Cao, Z.; He, W.-M. ACS Sustainable Chem. Eng. 2019, 7, 2169.
doi: 10.1021/cr000426w |
|
|
(h) Feng, C.-L.; Zhu, J.; Tang, Q.-J.; Zhou, A.-H. Chin. J. Org. Chem. 2019, 39, 1187.(in Chinese).
doi: 10.1021/cr000426w |
|
|
( 冯春来, 朱杰, 唐秋洁, 周爱华, 有机化学, 2019, 39, 1187.)
doi: 10.1021/cr000426w |
|
| [13] |
(a) Sun, K.; Wang, X.; Lv, Y.; Li, G.; Jiao, H.; Dai, C.; Li, Y.; Zhang, C.; Liu, L. Chem. Commun. 2016, 52, 8471.
doi: 10.1039/C6CC04225B |
|
(b) Sun, K.; ang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Green Chem. 2017, 19, 1490.
doi: 10.1039/C6CC04225B |
|
|
(c) Sun, K.; Lv, Y.; Shi, Z.; Fu, F.; Zhang, C.; Zhang, Z. Sci. China Chem. 2017, 60, 730.
doi: 10.1039/C6CC04225B |
|
|
(d) Sun, K.; Shi, Z.; Liu, Z.; Luan, B.; Zhu, J.; Xue, Y. Org. Lett. 2018, 20, 6687.
doi: 10.1039/C6CC04225B |
|
|
(e) Sun, K.; Wang, S.-N.; Feng, R.-R.; Zhang, Y.-X.; Wang, X.; Zhang, Z.-G.; Zhang, B. Org. Lett. 2019, 21, 2052.
doi: 10.1039/C6CC04225B |
|
| [14] |
Sun, K.; Wang, X.; Zhang, C.; Zhang, S.; Chen, Y.; Jiao, H.; Du, W. Chem. Asian J. 2017, 12, 713.
doi: 10.1002/asia.v12.6 |
| [15] |
For selected recent works, see: (a) Rafique, J.; Saba, S.; Rosário, A. R.; Braga, A.L. Chem.-Eur. J. 2016, 22, 11854.
doi: 10.1002/chem.201600800 |
|
(b) Sun, P.-F.; Jiang, M.; Wei, W.; Min, Y.-Y.; Zhang, W.; Li, W.-H. Yang, D.-S.; Wang, H. J. Org. Chem. 2017, 82, 2906.
doi: 10.1002/chem.201600800 |
|
|
(c) Guo, T.; Wei, X.-N.; Wang, H.-Y.; Zhu, Y.-L.; Zhao, Y.-H.; Ma, Y.-C. Org. Biomol. Chem. 2017, 15, 9455.
doi: 10.1002/chem.201600800 |
|
|
(d) Guo, T.; Dong, Z.; Zhang, P.-K.; Xing, W.-Q.; Li, L.-P. Tetrahedron Lett. 2018, 59, 2554.
doi: 10.1002/chem.201600800 |
|
|
(e) Zhu, J.; Zhu, W.-H.; Xie, P.; Pittman, C. U.; Zhou, A.-H. Tetrahedron 2018, 74, 6569.
doi: 10.1002/chem.201600800 |
|
|
(f) Rafique, J.; Saba, S.; Franco, M. S.; Bettanin, L.; Schneider, A. R.; Silva, L. T.; Braga, A.L. Chem.-Eur. J. 2018, 24, 4173.
doi: 10.1002/chem.201600800 |
|
|
(g) Guo, T.; Wei, X.-N.; Liu, Y.; Zhang, P.-K.; Zhao, Y.-H. Org. Chem. Front. 2019, 6, 1414.
doi: 10.1002/chem.201600800 |
|
| [16] |
For selected example with ionic liquid as the solvent, see: (a) Xie, L.-Y.; Peng, S.; Lu, L.-H.; Hu, J.; Bao, W.-H.; Zeng, F.; Tang, Z.; Xu, X.; He, W.-M. ACS Sustainable Chem. Eng. 2018, 6, 7989.
doi: 10.1021/acssuschemeng.8b01358 |
|
(b) Wu, C.; Lu, L.-H.; Peng, A.-Z.; Jia, G.-K.; Peng, C.; Cao, Z.; Tang, Z.; He, W.-M.; Xu, X. Green Chem. 2018, 20, 3683.
doi: 10.1021/acssuschemeng.8b01358 |
|
|
(c) Yang, G.-P.; Wu, X.; Yu, B.; Hu, C. ACS Sustainable Chem. Eng. 2019, 7, 3727.
doi: 10.1021/acssuschemeng.8b01358 |
| [1] | 殷一樊, 李晨, 孙凯, 刘颖杰, 王薪. 烯烃自由基胺硒化: β-氨基硒醚的简易合成[J]. 有机化学, 2022, 42(5): 1431-1437. |
| [2] | 李珊, 曹原, 蒋绿齐. 烷基、芳基和氟烷基硒化反应的研究进展[J]. 有机化学, 2022, 42(2): 434-457. |
| [3] | 易荣楠, 刘冬娴, 吴啟林, 赵明明, 王勇, 王峥. 电化学氧化-碘促进丙酮α-H芳(烷)硒化制备α-芳(烷)硒基丙酮[J]. 有机化学, 2021, 41(9): 3726-3732. |
| [4] | 孙名扬, 徐坤, 郭兵兵, 曾程初. 空气氧化的铜催化苯甲酸衍生物邻位C(sp2)—H键的硒化反应[J]. 有机化学, 2021, 41(6): 2302-2309. |
| [5] | 许颖, 李晨, 孟建萍, 黄玉玲, 付纪源, 刘冰, 刘颖杰, 陈宁. 有机硒参与的硒环化反应研究进展[J]. 有机化学, 2021, 41(3): 1012-1030. |
| [6] | 王玉超, 刘晋彪, 邱观音生, 杨宇, 周宏伟. N-羟乙基-N-芳基丙炔酰胺的无金属硒化螺三环化反应[J]. 有机化学, 2021, 41(12): 4798-4807. |
| [7] | 王薪, 张艳, 孙凯, 孟建萍, 张冰. 光电技术在含硒杂环合成中的应用研究[J]. 有机化学, 2021, 41(12): 4588-4609. |
| [8] | 何树华, 张行, 吴红谕, 周诗雨, 肖垚, 游贤会, 陈锦杨. ICl催化氨基香豆素衍生物Csp2—H芳(烷)硒化反应研究[J]. 有机化学, 2021, 41(11): 4378-4383. |
| [9] | 潘超, 刘鹏, 武安国, 李明, 文丽荣, 郭维斯. 电化学促进的N-烯丙基硫代酰胺的硒化/环化合成2-噻唑啉[J]. 有机化学, 2020, 40(9): 2855-2862. |
| [10] | 张继东, 詹妍, 李胡月雯, 齐怡, 王瑞鹏, 孟莉. 硒化合物荧光传感器研究进展[J]. 有机化学, 2020, 40(7): 1847-1859. |
| [11] | 葛颜玉, 孔晶, 杨成根, 杨倩, 张旭. 二(4-苄氧苯基)二硒醚的设计与制备:一种铜残留清除剂[J]. 有机化学, 2020, 40(6): 1760-1765. |
| [12] | 冯春来, 朱杰, 唐秋洁, 周爱华. Se粉为原料的芳硒基取代黄酮衍生物的合成[J]. 有机化学, 2019, 39(4): 1187-1192. |
| [13] | 陈亮, 王保取, 赵宇澄, 严胜骄, 林军. 一锅法合成多取代色酮并双环吡啶类化合物[J]. 有机化学, 2017, 37(6): 1433-1442. |
| [14] | 王小勇, 李治章, 张卫军, 王勰, 陈锦杨, 李宁波, 邱仁华, 许新华. 氢氧化铯催化端炔氢硒化: 高立体区域选择性合成(E)-1-芳硒基烯烃[J]. 有机化学, 2013, 33(03): 558-561. |
| [15] | 周建良, 刘建超, 陈启元. 咪唑并吡啶化合物的合成研究进展[J]. 有机化学, 2009, 29(11): 1708-1718. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||