有机化学 ›› 2021, Vol. 41 ›› Issue (6): 2485-2495.DOI: 10.6023/cjoc202011023 上一篇 下一篇
研究论文
李成飞, 岑波*( ), 段文贵*(
), 段文贵*( ), 林桂汕, 王秀, 李宝谕
), 林桂汕, 王秀, 李宝谕
收稿日期:2020-11-17
修回日期:2020-12-26
发布日期:2021-02-22
通讯作者:
岑波, 段文贵
基金资助:
Chengfei Li, Bo Cen( ), Wengui Duan(
), Wengui Duan( ), Guishan Lin, Xiu Wang, Baoyu Li
), Guishan Lin, Xiu Wang, Baoyu Li
Received:2020-11-17
Revised:2020-12-26
Published:2021-02-22
Contact:
Bo Cen, Wengui Duan
Supported by:文章分享
为了寻找以天然可再生资源为基础的除草剂, 设计并合成了26个新型含天然苯乙烯结构的4-甲基-1,2,4-三唑-硫醚类化合物, 并通过FTIR,1H NMR, 13C NMR, ESI-MS和元素分析等方法对其结构进行了确认. 初步的除草活性测试表明, 在质量浓度为100 µg/mL时, 大多数目标化合物对油菜胚根生长表现出良好的抑制活性, 其中8个化合物的抑制率超过81.4%, 远优于阳性对照丙炔氟草胺(抑制率63.0%); 2个化合物对稗草幼苗生长有良好的抑制活性. 比较发现, R为脂肪族取代基或吡啶环对除草活性有利. 此外, 利用CoMFA方法对R为芳族取代基的目标化合物的油菜胚根抑制活性进行了初步的三维定量构效关系(3D-QSAR)研究, 建立了合理有效的3D-QSAR模型( r2=0.996, q2=0.603).
李成飞, 岑波, 段文贵, 林桂汕, 王秀, 李宝谕. 含天然苯乙烯结构的4-甲基-1,2,4-三唑-硫醚化合物的合成、除草活性及三维定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(6): 2485-2495.
Chengfei Li, Bo Cen, Wengui Duan, Guishan Lin, Xiu Wang, Baoyu Li. Synthesis, Herbicidal Activity and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Study of 4-Methyl- 1,2,4-triazole-thioether Compounds Containing Natural Styrene Structure[J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2485-2495.
| Compd. | B. campestris | E. crusgalli | |||
|---|---|---|---|---|---|
| 10 μg/mL | 100 μg/mL | 10 μg/mL | 100 μg/mL | ||
| Flumioxazin | 57.8 | 63.0 | 95.1 | 97.5 | |
| 5a | 30.7 | 74.9 | 5.0 | 20.0 | |
| 5b | 68.9 | 86.9 | 15.0 | 40.0 | |
| 5c | 79.3 | 89.4 | 25.0 | 40.0 | |
| 5d | 41.7 | 90.5 | 0 | 0 | |
| 5e | 67.5 | 83.2 | 0 | 0 | |
| 5f | 38.3 | 76.8 | 0 | 0 | |
| 5g | 22.6 | 75.7 | 10.0 | 40.0 | |
| 5h | 25.3 | 77.4 | 35.0 | 70.0 | |
| 5i | 38.9 | 81.4 | 15.0 | 75.0 | |
| 5j | 10.0 | 47.9 | 20.0 | 50.0 | |
| 5k | 0 | 79.3 | 20.0 | 40.0 | |
| 5l | 34.0 | 73.0 | 35.0 | 55.0 | |
| 5m | 13.8 | 65.1 | 15.0 | 30.0 | |
| 5n | 4.6 | 31.8 | 10.0 | 25.0 | |
| 5o | 25.8 | 60.5 | 10.0 | 25.0 | |
| 5p | 4.6 | 18.7 | 0 | 0 | |
| 5q | 20.9 | 81.7 | 15.0 | 20.0 | |
| 5r | 29.6 | 52.3 | 20.0 | 50.0 | |
| 5s | 0 | 9.0 | 0 | 10.0 | |
| 5t | 23.7 | 57.8 | 0 | 0 | |
| 5u | 21.1 | 25.2 | 0 | 10.0 | |
| 5v | 37.2 | 79.1 | 0 | 0 | |
| 5w | 24.5 | 55.1 | 0 | 10.0 | |
| 5x | 60.7 | 83.2 | 0 | 10.0 | |
| 5y | 45.0 | 60.2 | 0 | 0 | |
| 5z | 30.8 | 85.6 | 0 | 0 | |
| Compd. | B. campestris | E. crusgalli | |||
|---|---|---|---|---|---|
| 10 μg/mL | 100 μg/mL | 10 μg/mL | 100 μg/mL | ||
| Flumioxazin | 57.8 | 63.0 | 95.1 | 97.5 | |
| 5a | 30.7 | 74.9 | 5.0 | 20.0 | |
| 5b | 68.9 | 86.9 | 15.0 | 40.0 | |
| 5c | 79.3 | 89.4 | 25.0 | 40.0 | |
| 5d | 41.7 | 90.5 | 0 | 0 | |
| 5e | 67.5 | 83.2 | 0 | 0 | |
| 5f | 38.3 | 76.8 | 0 | 0 | |
| 5g | 22.6 | 75.7 | 10.0 | 40.0 | |
| 5h | 25.3 | 77.4 | 35.0 | 70.0 | |
| 5i | 38.9 | 81.4 | 15.0 | 75.0 | |
| 5j | 10.0 | 47.9 | 20.0 | 50.0 | |
| 5k | 0 | 79.3 | 20.0 | 40.0 | |
| 5l | 34.0 | 73.0 | 35.0 | 55.0 | |
| 5m | 13.8 | 65.1 | 15.0 | 30.0 | |
| 5n | 4.6 | 31.8 | 10.0 | 25.0 | |
| 5o | 25.8 | 60.5 | 10.0 | 25.0 | |
| 5p | 4.6 | 18.7 | 0 | 0 | |
| 5q | 20.9 | 81.7 | 15.0 | 20.0 | |
| 5r | 29.6 | 52.3 | 20.0 | 50.0 | |
| 5s | 0 | 9.0 | 0 | 10.0 | |
| 5t | 23.7 | 57.8 | 0 | 0 | |
| 5u | 21.1 | 25.2 | 0 | 10.0 | |
| 5v | 37.2 | 79.1 | 0 | 0 | |
| 5w | 24.5 | 55.1 | 0 | 10.0 | |
| 5x | 60.7 | 83.2 | 0 | 10.0 | |
| 5y | 45.0 | 60.2 | 0 | 0 | |
| 5z | 30.8 | 85.6 | 0 | 0 | |
| Compd. | ED | ED'' | Residue |
|---|---|---|---|
| 5f | –1.97 | –2.03 | 0.06 |
| 5g | –2.01 | –2.00 | –0.01 |
| 5h | –1.97 | –1.97 | 0 |
| 5i | –1.89 | –1.90 | 0.01 |
| 5j | –2.56 | –2.56 | 0 |
| 5k | –1.93 | –1.90 | –0.03 |
| 5l | –2.08 | –2.03 | –0.05 |
| 5m | –2.24 | –2.23 | –0.01 |
| 5n | –2.86 | –2.84 | –0.02 |
| 5o | –2.35 | –2.32 | –0.03 |
| 5s | –3.55 | –3.55 | 0 |
| 5t | –2.47 | –2.45 | –0.02 |
| 5u | –3.03 | –3.05 | 0.02 |
| 5v | –1.96 | –2.01 | 0.05 |
| 5w | –2.49 | –2.52 | 0.03 |
| 5p* | –3.17 | –3.06 | –0.11 |
| 5q* | –1.94 | –1.98 | 0.04 |
| 5r* | –2.55 | –2.64 | 0.09 |
| Compd. | ED | ED'' | Residue |
|---|---|---|---|
| 5f | –1.97 | –2.03 | 0.06 |
| 5g | –2.01 | –2.00 | –0.01 |
| 5h | –1.97 | –1.97 | 0 |
| 5i | –1.89 | –1.90 | 0.01 |
| 5j | –2.56 | –2.56 | 0 |
| 5k | –1.93 | –1.90 | –0.03 |
| 5l | –2.08 | –2.03 | –0.05 |
| 5m | –2.24 | –2.23 | –0.01 |
| 5n | –2.86 | –2.84 | –0.02 |
| 5o | –2.35 | –2.32 | –0.03 |
| 5s | –3.55 | –3.55 | 0 |
| 5t | –2.47 | –2.45 | –0.02 |
| 5u | –3.03 | –3.05 | 0.02 |
| 5v | –1.96 | –2.01 | 0.05 |
| 5w | –2.49 | –2.52 | 0.03 |
| 5p* | –3.17 | –3.06 | –0.11 |
| 5q* | –1.94 | –1.98 | 0.04 |
| 5r* | –2.55 | –2.64 | 0.09 |
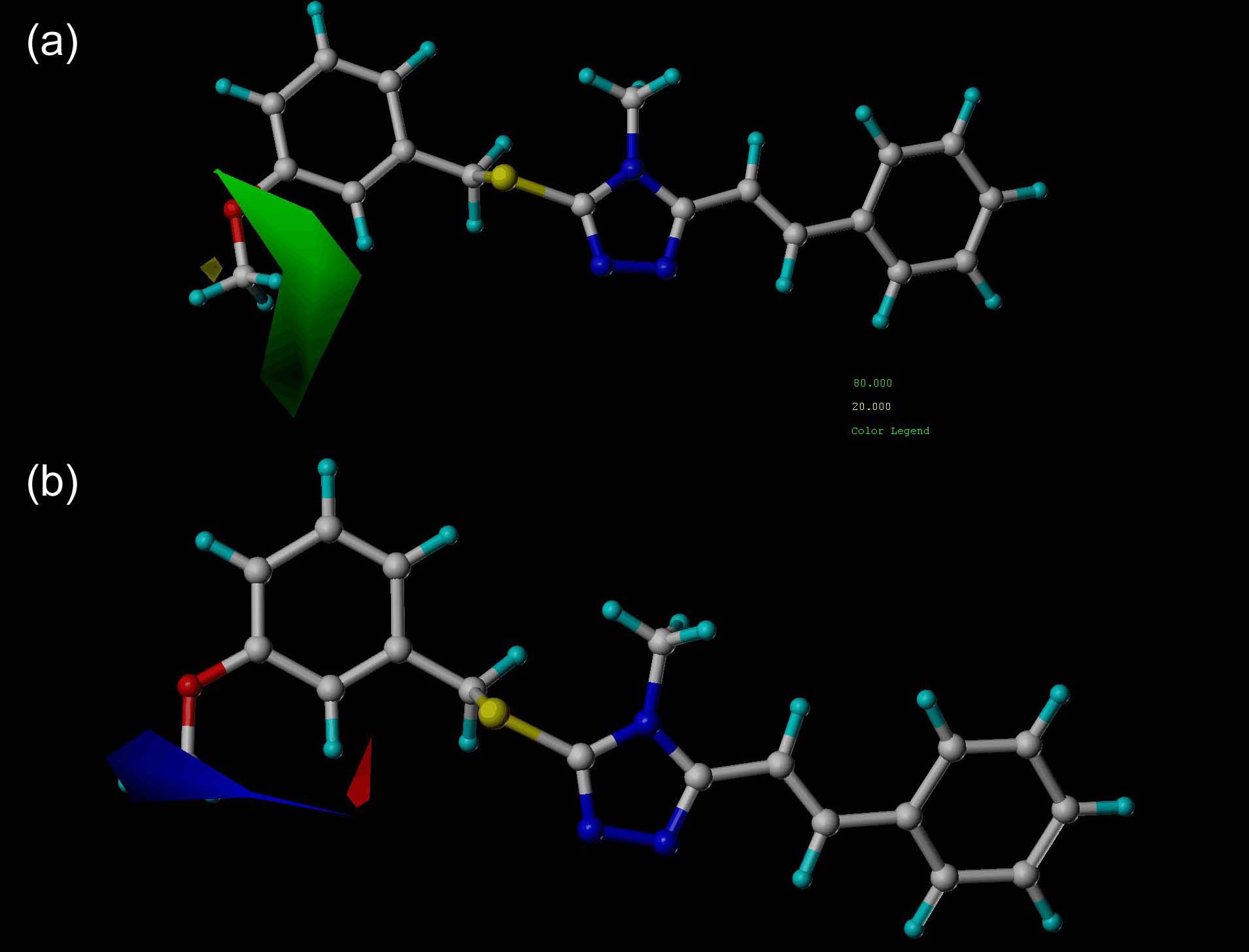


| [1] |
Yang, L.; Zhou, L. Z.; Chen, H. Y.; Gu, Y. Farm. Prod. Process 2019, 2,41(in Chinese).
|
|
( 杨漓, 周丽珠, 陈海燕, 谷瑶, 农产品加工, 2019, 2,41. )
|
|
| [2] |
Wu, C. H.; Shu, M.; Li, Q.; Ding, P. Chin. Med. J. Res. Prac. 2017, 31,14(in Chinese).
|
|
( 伍彩红, 舒眉, 李倩, 丁平, 现代中药研究与实践, 2017, 31,14.)
|
|
| [3] |
Li, Q. Z.; Song, B. A.; Cai, X. J.; Zheng, Y. G.; Guo, Q. Q. Chin. J. Org. Chem. 2010, 30,569(in Chinese).
|
|
( 李黔柱, 宋宝安, 蔡学建, 郑玉国, 郭晴晴, 有机化学, 2010, 30,569.)
|
|
| [4] |
Zhang, X. B.; Ma, H. Y.; Sun, T. D.; Lei, P.; Yang, X. L.; Zhang, X. M.; Ling, Y. Chin. J. Org. Chem. 2019, 39,2965(in Chinese).
doi: 10.6023/cjoc201903031 |
|
( 张学博, 马航宇, 孙腾达, 雷鹏, 杨新玲, 张晓鸣, 凌云, 有机化学, 2019, 39,2965.)
doi: 10.6023/cjoc201903031 |
|
| [5] |
Marona, H.; Szkaradek, N.; Karczewska, E.; Trojanowska, D.; Budak, A.; Bober, P.; Przepiorka, W.; Marek Cegla, M.; Szneler, E. Arch. Pharm. Chem. Life Sci. 2010, 342,9.
doi: 10.1002/ardp.v342:1 |
| [6] |
Xu, Y.; Lei, P.; Ling, Y.; Wang, S. W.; Yang, X. L. Chin. J. Org. Chem. 2014, 34,1118(in Chinese).
doi: 10.6023/cjoc201401034 |
|
( 徐焱, 雷鹏, 凌云, 王圣文, 杨新玲, 有机化学, 2014, 34,1118.)
doi: 10.6023/cjoc201401034 |
|
| [7] |
Friedman, M. J. Agric. Food Chem. 2017, 65,10406.
doi: 10.1021/acs.jafc.7b04344 |
| [8] |
Li, X.; sheng, J. Z.; Huang, G. H.; Ma, R. X.; Yin, F. X.; Song, D.; Zhao, C.; Ma, S. T. Eur. J. Med. Chem. 2015, 97,32.
doi: 10.1016/j.ejmech.2015.04.048 |
| [9] |
Kim, H. K.; Kim, J. R.; Ahn, Y. J. J. Stored. Prod. Res. 2004, 40,55.
doi: 10.1016/S0022-474X(02)00075-9 |
| [10] |
Saad, M. M. G.; Gouda, N. A. A.; Abdelgaleil, S. A. M. J. Environ. Sci. Heal. B 2019, 54,954.
doi: 10.1080/03601234.2019.1653121 |
| [11] |
Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Molecules 2018, 23,471.
doi: 10.3390/molecules23020471 |
| [12] |
Muñoz, M.; Torres‐Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez‐Moreiras, A. M.; Verdeguer, M. Agronomy 2020, 10,791.
doi: 10.3390/agronomy10060791 |
| [13] |
Zhou, J.; Yuan, X. R.; Li, L.; Zhang, T.; Wang, B. Nat. Prod. Res. 2017, 31,2909.
doi: 10.1080/14786419.2017.1299724 |
| [14] |
Kwon, B. M.; Lee, S. H.; Choi, S. U.; Park, S. H.; lee, C. O.; Cho, Y. K.; Sung, N. D.; Bok, S. H. Arch. Pharm. Res. 1998, 21,147.
pmid: 9875422 |
| [15] |
Ka, H.; Park, H. J.; Jung, H. J.; Choi, J. W.; Cho, K. S.; Ha, J.; Lee, K. T. Cancer Lett. 2003, 196,143.
doi: 10.1016/S0304-3835(03)00238-6 |
| [16] |
Cabello, C. M.; 3rd, W. B. B.; Lamore, S. D.; Ley, S.; Bause, A. S.; Azimian, S.; Wondrak, G.T.. Free Radic. Biol. Med. 2009, 46,220.
doi: 10.1016/j.freeradbiomed.2008.10.025 |
| [17] |
Wu, W. N.; Jiang, Y. M.; Zhang, B. Y.; Wang, J. Z.; Du, H. T.; Fei, Q. Chemistry 2019, 82,1115(in Chinese).
|
|
( 吴文能, 姜阳明, 张卜艳, 王家忠, 杜海堂, 费强, 化学通报, 2019, 82,1115.)
|
|
| [18] |
He, S. C.; Zhang, H. Z.; Zhang, H. J.; Sun, Q.; Zhou, C. H. Med. Chem. 2020, 16,104.
doi: 10.2174/1573406414666181106124852 |
| [19] |
Liu, X. H.; Sun, Z. H.; Yang, M. Y.; Tan, C. X.; Weng, J. Q.; Zhang, Y. G.; Ma, Y. Chem. Biol. Drug Des. 2014, 84,342.
doi: 10.1111/cbdd.2014.84.issue-3 |
| [20] |
Rode, N. D.; Sonawane, A. D.; Nawale, L.; Khedkar, V. M.; Joshi, R. A.; Likhite, A. P.; Sarkar, D.; Joshi, R. R. Chem. Biol. Drug Des. 2017, 90,1206.
doi: 10.1111/cbdd.2017.90.issue-6 |
| [21] |
Gilandoust, M.; Harsha, K. B.; Mohan, C. D.; Raquib, A. R.; Rangappa, S.; Pandey, V.; Lobie, P. E.; Basappa
doi: S0960-894X(18)30420-7 pmid: 29789259 |
| [22] |
Korcz, M.; Saczewski, F.; Bednarski, P. J.; Kornicka, A. Molecules 2018, 23,1497.
doi: 10.3390/molecules23061497 |
| [23] |
Massari, S.; Nannetti, G.; Desantis, J.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Palu, G.; Cruciani, G.; Loregian, A.; Goracci, L.; Tabarrini, O. J. Med. Chem. 2015, 58,3830.
doi: 10.1021/acs.jmedchem.5b00012 pmid: 25856229 |
| [24] |
Liu, X. H.; Xu, X. Y.; Tan, C. X.; Weng, J. Q.; Xin, J. H.; Chen, J. Pest Manage. Sci. 2015, 71,292.
doi: 10.1002/ps.3804 |
| [25] |
Wang, B. L.; Zhang, L. Y.; Liu, X. H.; Ma, Y.; Zhang, Y.; Li, Z. M.; Zhang, X. Bioorg. Med. Chem. Lett. 2017, 27,5457.
doi: 10.1016/j.bmcl.2017.10.065 |
| [26] |
Wang, B. L.; Zhang, L. Y.; Zhan, Y. Z.; Zhang, Y.; Zhang, X.; Wang, L. Z.; Li, Z. M. J. Fluorine Chem. 2016, 184,36.
doi: 10.1016/j.jfluchem.2016.02.004 |
| [27] |
Xu, W. M.; Song, B. A.; Yang, S.; Hu, D. Y.; Zeng, S. Agrochemicals 2010, 49,625(in Chinese).
|
|
( 徐维明, 宋宝安, 杨松, 胡德禹, 曾松, 农药, 2010, 49,625.)
|
|
| [28] |
HRAC Classification on Mode of Action 2020. (accessed September 26, 2020). https://www.hracglobal.com.
|
| [29] |
Lu, X. L.; Zhu, X.; Zhang, M.; Wu, Q. L.; Zhou, X. D.; Li, J. K. Nat. Prod. Lett. 2019, 33,2145.
|
| [30] |
Cheng, C. R.; Zheng, Z.; Liang, R. M.; Li, F.; X. Jiang, Q. Q.; Yue, L.; Wang, Q.; Ding, J.; Liu, Y.. Chem. Nat. Compd. 2020, 56,264.
doi: 10.1007/s10600-020-03003-4 |
| [31] |
Chen, Y.; Li, P.; Chen, M.; He, J.; Su, S. J.; He, M.; Wang, H.; Xue, W. J. Heterocycl. Chem. 2020, 57,1.
doi: 10.1002/jhet.v57.1 |
| [32] |
Ruan, X. H.; Zhang, C.; Jiang, S. C.; Guo, T.; Xia, R. J.; Chen, Y.; Tang, X.; Xue, W. Molecules 2018, 23,3132.
doi: 10.3390/molecules23123132 |
| [33] |
Barrows, R. D.; Hammill, J. T.; Michael, C. Tran, M. C.; Falade, M. O.; Rice, A. L.; Davis, C. W.; Emge, T. J.; Rablen, P. R.; Guy, R. K.; Knapp, S. Bioorg. Med. Chem. 2020 , 28, 115758..
|
| [34] |
Guo, Y.; Wang, X. G.; Fan, J. P.; Zhang, Q.; Wang, Y.; Zhao, Y.; Huang, M. X.; Ding, M.; Zhang, Y. B. Roy. Soc. Open Sci. 2017, 4,171053.
|
| [35] |
Yu, Y. P.; Duan, W. G.; Lin, G. S.; Kang, G. Q.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2020, 40,1647(in Chinese).
doi: 10.6023/cjoc201912042 |
|
( 虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚, 有机化学, 2020, 40,1647.)
doi: 10.6023/cjoc201912042 |
|
| [36] |
Li, F. Y.; Huang, L.; Zhou, X. Q.; Li, Q.; Ma, X. L.; Duan, W. G.; Wang, X. Chin. J. Org. Chem. 2020, 40,2845(in Chinese).
doi: 10.6023/cjoc202003062 |
|
( 李芳耀, 黄琳, 周小群, 李倩, 马献力, 段文贵, 王秀, 有机化学, 2020, 40,2845.)
doi: 10.6023/cjoc202003062 |
|
| [37] |
Lin, G. S.; Chen, Z. C.; Duan, W. G.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2018, 38,2085(in Chinese).
doi: 10.6023/cjoc201801043 |
|
( 林桂汕, 陈智聪, 段文贵, 王晓宇, 雷福厚, 有机化学, 2018, 38,2085.)
doi: 10.6023/cjoc201801043 |
|
| [38] |
He, Y.; Duan, W. G.; Lin, G. S.; Cen, B.; Bu, J. W.; Lei, F. H. Chem. Ind. Forest Prod. 2020, 40,76(in Chinese).
|
|
( 何云, 段文贵, 林桂汕, 岑波, 卜俊文, 雷福厚, 林产化学与工业, 2020, 40,76.)
|
|
| [39] |
Lin, G. S.; Bai, X.; Duan, W. G.; Cen, B.; Huang, M.; Lu, S. Z. ACS Sustainable Chem. Eng. 2019, 7,7862.
doi: 10.1021/acssuschemeng.9b00254 |
| [40] |
Lin, G. S.; Duan, W. G.; Yang, L. X.; Huang, M.; Lei, F. H. Molecules 2017, 22,193.
doi: 10.3390/molecules22020193 |
| [41] |
Verma, J.; Khedkar, V. M.; Coutinho, E. C. Curr. Top. Med. Chem. 2010, 10,95.
doi: 10.2174/156802610790232260 |
| [42] |
Li, L. H.; Li, Z. R.; Liu, M. L.; Shen, W. Y.; Wang, B.; Guo, H. Y.; Lu, Y. Molecules 2015, 21,49.
doi: 10.3390/molecules21010049 |
| [43] |
Liu, M.X; Li, C. J.. Angew. Chem. Int. Ed. 2016, 55,10806.
doi: 10.1002/anie.201604847 |
| [44] |
Li, L. H.; Li, Z. R.; Liu, M. L.; Shen, W. Y.; Wang, B.; Guo, H. Y.; Lu, Y. Molecules 2016, 21,49.
doi: 10.3390/molecules21010049 |
| [45] |
Su, N. N.; Li, Y.; Yu, S. J.; Zhang, X.; Liu, X. H.; Zhao, W. G. Res. Chem. Intermediat. 2012, 39,759.
doi: 10.1007/s11164-012-0595-9 |
| [1] | 曾崇洋, 胡平, 汪必琴, 方文彦, 赵可清. 氰基二苯乙烯桥联苯并菲二联体刺激响应盘状液晶: 合成、性质与应用[J]. 有机化学, 2023, 43(9): 3287-3296. |
| [2] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| [3] | 林海, 聂会祥, 赵安林, 王涛, 罗劲. 新型嘧啶并[5,4-e][1,2,4]三唑并[1,5-c]嘧啶衍生物的合成与除草活性[J]. 有机化学, 2023, 43(7): 2462-2475. |
| [4] | 刘铃, 浩涛涛, 伍晚花, 杨成. 利用超分子策略构筑具有聚集诱导发光(AIE)功能的二苯乙烯型分子开关[J]. 有机化学, 2023, 43(6): 2189-2196. |
| [5] | 李进京, 孙立娇, 赵岩, 史成阳. β-硝基苯乙烯参与的反应研究进展[J]. 有机化学, 2023, 43(12): 4168-4187. |
| [6] | 余富欢, 周志宽, 谢威, 周传庭, 盖立志, 卢华. 硅烷桥联四苯乙烯-寡聚噻吩衍生物的结构与光谱性质[J]. 有机化学, 2023, 43(10): 3652-3660. |
| [7] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| [8] | 张继东, 颜婉琳, 胡文强, 郭典, 张大龙, 权校昕, 卜贤盼, 陈思宇. 一种具有聚集诱导发光性能的Zn2+荧光探针的设计合成[J]. 有机化学, 2023, 43(1): 326-331. |
| [9] | 李嘉欣, 贺如艳, 段森林, 黎锦华, 韩校净, 叶勇. 一种检测ONOO–的新型荧光探针的构建与细胞成像研究[J]. 有机化学, 2022, 42(8): 2428-2432. |
| [10] | 石发胜, 王圣文, 徐欢, 路星星, 杨新玲, 孙腾达, 王长凯, 张晓鸣, 杨青, 凌云. 新型缩氨基硫脲类化合物的设计、合成及杀菌活性研究[J]. 有机化学, 2022, 42(7): 2106-2116. |
| [11] | 崔玉成, 陈美桦, 林桂汕, 段文贵, 李晴敏, 邹壬萱, 岑波. 含偕二甲基环丙烷结构的1,3,4-噻二唑-脲化合物的合成、抑菌活性及分子对接研究[J]. 有机化学, 2022, 42(11): 3784-3797. |
| [12] | 李芯颖, 赵志翔, 胡林海, 魏登澈, 柳清湘. 基于四苯乙烯的四齿唑盐: 合成和对离子的选择性识别[J]. 有机化学, 2021, 41(9): 3608-3616. |
| [13] | 王超超, 刘会, 赵微, 李攀, 冀庐莎, 柳仁民, 雷康, 徐效华. 5-(1-氨基-2-苯氧亚乙基)巴比妥酸衍生物的合成及除草活性研究[J]. 有机化学, 2021, 41(5): 2063-2073. |
| [14] | 何淑旺, 颜世强, 郭伟, 翟光喜, 张伟. 苯乙烯一锅法合成氨基醇[J]. 有机化学, 2020, 40(7): 2094-2098. |
| [15] | 虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚. 新型含偕二甲基环丙烷的4-甲基-1,2,4-三唑硫醚化合物的合成、生物活性及三维定量构效关系(3D-QSAR)研究[J]. 有机化学, 2020, 40(6): 1647-1657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||