有机化学 ›› 2021, Vol. 41 ›› Issue (8): 3157-3170.DOI: 10.6023/cjoc202102046 上一篇 下一篇
研究论文
李英俊a,*( ), 林乐弟a, 靳焜b, 高立信c, 盛丽c, 刘季红d, 李佳c,*(
), 林乐弟a, 靳焜b, 高立信c, 盛丽c, 刘季红d, 李佳c,*( )
)
收稿日期:2021-02-24
修回日期:2021-04-02
发布日期:2021-05-14
通讯作者:
李英俊, 李佳
基金资助:
Yingjun Lia( ), Ledi Lina, Kun Jinb, Lixin Gaoc, Li Shengc, Jihong Liud, Jia Lic(
), Ledi Lina, Kun Jinb, Lixin Gaoc, Li Shengc, Jihong Liud, Jia Lic( )
)
Received:2021-02-24
Revised:2021-04-02
Published:2021-05-14
Contact:
Yingjun Li, Jia Li
Supported by:文章分享

为寻找新型蛋白酪氨酸磷酸酶1B (PTP1B)抑制剂, 设计并合成了一系列新型含咔唑环芳氨基乙酰腙衍生物. 其结构和构型用IR、1H NMR、13C NMR和2D NMR(包括1H-1H COSY、1H-13C HMBC和NOESY)谱及元素分析进行了确证. 通过对PTP1B抑制活性的测试发现, 目标化合物对PTP1B有较强的抑制作用, 且大多数化合物的IC50值低于阳性对照药物齐墩果酸, 其中N'-(9-辛基咔唑-3-亚甲基)-2-(4-硝基苯氨基)乙酰肼(3t)活性最高, IC50=(2.78±0.04) μmol/L. 利用分子对接研究了化合物3t与PTP1B酶的结合情况.
李英俊, 林乐弟, 靳焜, 高立信, 盛丽, 刘季红, 李佳. 新型含咔唑环芳氨基乙酰腙衍生物的合成及其蛋白酪氨酸磷酸酶1B (PTP1B)抑制活性评价[J]. 有机化学, 2021, 41(8): 3157-3170.
Yingjun Li, Ledi Lin, Kun Jin, Lixin Gao, Li Sheng, Jihong Liu, Jia Li. Synthesis and Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Activity Evaluation of Novel Arylaminoacetylhydrazone Derivatives Containing Carbazole Moiety[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3157-3170.


| Position | δH | 1H-1H COSY A (B) | NOESY A (B) | |
|---|---|---|---|---|
| A | B | |||
| 1 (1') | 7.65 (d, J=8.5 Hz) | — | H-2 (—) | H-16 (—) |
| 2 (2') | 7.92 (dd, J=8.5, 1.0 Hz) | 7.87 (dd, J=8.5, 1.0 Hz) | H-1,4 (H-4') | H-9/H-9' |
| 3 (3') | / | / | / | / |
| 4 (4') | 8.48 | 8.46 | H-2 (—) | / |
| 5 (5') | 8.25 (d, J=7.5 Hz) | 8.21 (d, J=7.5 Hz) | H-6 (H-6') | / |
| 6 (6') | 7.244 (t, J=7.5 Hz) | 7.237 (t, J=7.5 Hz) | H-5,7 (H-5') | / |
| 7 (7') | 7.48 (t, J=7.0 Hz) | — | H-6,8 (—) | / |
| 8 (8') | 7.61 (d, J=8.0 Hz) | — | H-7 (—) | H-16 (—) |
| 9 (9') | 8.22 | 8.41 | / | H-2/H-2'/H-10/H-10' |
| 10 (10') | 11.59 | 11.56 | / | H-9/H-9'/–/H-12' |
| 11 (11') | / | / | / | / |
| 12 (12') | 4.51 (d, J=6.0 Hz) | 4.04 (d, J=6.0 Hz) | H-13 (—) | (—)/H-10'/H-14/H-14' |
| 13 (13') | 7.47 | — | H-12 (—) | H-14 (—) |
| 14 (14') | 6.79 (d, J=9.0 Hz) | 6.74 (d, J=9.0 Hz) | H-15 (H-15') | H-12/H-12'/H-13 |
| 15 (15') | 8.04 (d, J=9.0 Hz) | 8.06 (d, J=9.0 Hz) | H-14 (H-14') | / |
| 16 (16') | 4.40 (t, J=6.5 Hz) | 4.38 (t, J=6.5 Hz) | / | H-1/H-8 |
| Position | δH | 1H-1H COSY A (B) | NOESY A (B) | |
|---|---|---|---|---|
| A | B | |||
| 1 (1') | 7.65 (d, J=8.5 Hz) | — | H-2 (—) | H-16 (—) |
| 2 (2') | 7.92 (dd, J=8.5, 1.0 Hz) | 7.87 (dd, J=8.5, 1.0 Hz) | H-1,4 (H-4') | H-9/H-9' |
| 3 (3') | / | / | / | / |
| 4 (4') | 8.48 | 8.46 | H-2 (—) | / |
| 5 (5') | 8.25 (d, J=7.5 Hz) | 8.21 (d, J=7.5 Hz) | H-6 (H-6') | / |
| 6 (6') | 7.244 (t, J=7.5 Hz) | 7.237 (t, J=7.5 Hz) | H-5,7 (H-5') | / |
| 7 (7') | 7.48 (t, J=7.0 Hz) | — | H-6,8 (—) | / |
| 8 (8') | 7.61 (d, J=8.0 Hz) | — | H-7 (—) | H-16 (—) |
| 9 (9') | 8.22 | 8.41 | / | H-2/H-2'/H-10/H-10' |
| 10 (10') | 11.59 | 11.56 | / | H-9/H-9'/–/H-12' |
| 11 (11') | / | / | / | / |
| 12 (12') | 4.51 (d, J=6.0 Hz) | 4.04 (d, J=6.0 Hz) | H-13 (—) | (—)/H-10'/H-14/H-14' |
| 13 (13') | 7.47 | — | H-12 (—) | H-14 (—) |
| 14 (14') | 6.79 (d, J=9.0 Hz) | 6.74 (d, J=9.0 Hz) | H-15 (H-15') | H-12/H-12'/H-13 |
| 15 (15') | 8.04 (d, J=9.0 Hz) | 8.06 (d, J=9.0 Hz) | H-14 (H-14') | / |
| 16 (16') | 4.40 (t, J=6.5 Hz) | 4.38 (t, J=6.5 Hz) | / | H-1/H-8 |
| Compd. | X | R | PTP1Ba | |
|---|---|---|---|---|
| Inhibition/% at 20 μg/mL | IC50/(μmol•L–1) | |||
| 3a | 4-OCH3 | C2H5 | 93.19±1.27 | 9.91±1.72 |
| 3b | 4-OCH3 | n-C3H7 | 93.92±1.48 | 9.00±0.63 |
| 3c | 4-OCH3 | n-C4H9 | 91.82±0.32 | 9.31±0.16 |
| 3d | 4-OCH3 | n-C5H11 | 81.15±2.86 | 7.68±1.56 |
| 3e | 4-OCH3 | n-C6H13 | 76.38±10.73 | 5.26±1.66 |
| 3f | 4-OCH3 | n-C7H15 | 74.15±1.24 | 5.38±2.74 |
| 3g | 4-OCH3 | n-C8H17 | 53.68±0.33 | 7.76±0.54 |
| 3h | 3-Cl | C2H5 | 90.21±1.19 | 7.09±1.11 |
| 3i | 3-Cl | n-C3H7 | 95.32±0.16 | 5.80±0.72 |
| 3j | 3-Cl | n-C4H9 | 95.24±0.15 | 3.88±0.02 |
| 3k | 3-Cl | n-C5H11 | 96.46±0.24 | 6.67±0.18 |
| 3l | 3-Cl | n-C6H13 | 96.03±1.67 | 4.14±0.13 |
| 3m | 3-Cl | n-C7H15 | 93.34±0.21 | 4.51±0.23 |
| 3n | 3-Cl | n-C8H17 | 83.08±3.72 | 5.48±2.58 |
| 3o | 4-NO2 | n-C3H7 | 77.04±1.71 | 5.59±0.98 |
| 3p | 4-NO2 | n-C4H9 | 74.05±2.13 | 4.08±1.38 |
| 3q | 4-NO2 | n-C5H11 | 73.23±0.85 | 5.57±1.33 |
| 3r | 4-NO2 | n-C6H13 | 95.37±1.33 | 4.41±1.17 |
| 3s | 4-NO2 | n-C7H15 | 96.39±0.24 | 3.27±0.47 |
| 3t | 4-NO2 | n-C8H17 | 96.70±1.63 | 2.78±0.04 |
| Compd. | X | R | PTP1Ba | |
|---|---|---|---|---|
| Inhibition/% at 20 μg/mL | IC50/(μmol•L–1) | |||
| 3a | 4-OCH3 | C2H5 | 93.19±1.27 | 9.91±1.72 |
| 3b | 4-OCH3 | n-C3H7 | 93.92±1.48 | 9.00±0.63 |
| 3c | 4-OCH3 | n-C4H9 | 91.82±0.32 | 9.31±0.16 |
| 3d | 4-OCH3 | n-C5H11 | 81.15±2.86 | 7.68±1.56 |
| 3e | 4-OCH3 | n-C6H13 | 76.38±10.73 | 5.26±1.66 |
| 3f | 4-OCH3 | n-C7H15 | 74.15±1.24 | 5.38±2.74 |
| 3g | 4-OCH3 | n-C8H17 | 53.68±0.33 | 7.76±0.54 |
| 3h | 3-Cl | C2H5 | 90.21±1.19 | 7.09±1.11 |
| 3i | 3-Cl | n-C3H7 | 95.32±0.16 | 5.80±0.72 |
| 3j | 3-Cl | n-C4H9 | 95.24±0.15 | 3.88±0.02 |
| 3k | 3-Cl | n-C5H11 | 96.46±0.24 | 6.67±0.18 |
| 3l | 3-Cl | n-C6H13 | 96.03±1.67 | 4.14±0.13 |
| 3m | 3-Cl | n-C7H15 | 93.34±0.21 | 4.51±0.23 |
| 3n | 3-Cl | n-C8H17 | 83.08±3.72 | 5.48±2.58 |
| 3o | 4-NO2 | n-C3H7 | 77.04±1.71 | 5.59±0.98 |
| 3p | 4-NO2 | n-C4H9 | 74.05±2.13 | 4.08±1.38 |
| 3q | 4-NO2 | n-C5H11 | 73.23±0.85 | 5.57±1.33 |
| 3r | 4-NO2 | n-C6H13 | 95.37±1.33 | 4.41±1.17 |
| 3s | 4-NO2 | n-C7H15 | 96.39±0.24 | 3.27±0.47 |
| 3t | 4-NO2 | n-C8H17 | 96.70±1.63 | 2.78±0.04 |

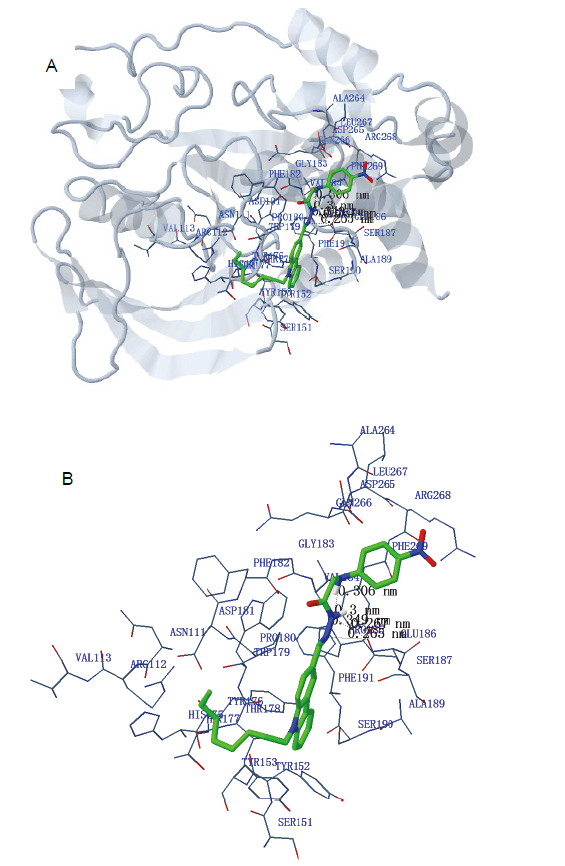
| Est. free energy/ (kJ/mol) of binding | No. of H-bond | H-bond (length/nm) | Polar (length/nm) | Hydrophobic (length/nm) | π-π (length/nm) |
|---|---|---|---|---|---|
| –25.75 | 5 | N(2)-VAL184 (0.349) | H(26)-GLU186 (0.245) | C(9)-PRO180 (0.352) | C(4)-TYR152 (0.382) |
| N(3)-VAL184 (0.300) | O(2)-ARG268 (0.370) | C(10)-PRO180 (0.314) | |||
| N(4)-VAL184 (0.306) | O(3)-ARG268 (0.336) | C(11)-PRO180 (0.355) | |||
| N(2)-GLU186 (0.265) | N(5)-ARG268 (0.387) | C(21)-PRO180 (0.324) | |||
| N(3)-GLU186 (0.267) |
| Est. free energy/ (kJ/mol) of binding | No. of H-bond | H-bond (length/nm) | Polar (length/nm) | Hydrophobic (length/nm) | π-π (length/nm) |
|---|---|---|---|---|---|
| –25.75 | 5 | N(2)-VAL184 (0.349) | H(26)-GLU186 (0.245) | C(9)-PRO180 (0.352) | C(4)-TYR152 (0.382) |
| N(3)-VAL184 (0.300) | O(2)-ARG268 (0.370) | C(10)-PRO180 (0.314) | |||
| N(4)-VAL184 (0.306) | O(3)-ARG268 (0.336) | C(11)-PRO180 (0.355) | |||
| N(2)-GLU186 (0.265) | N(5)-ARG268 (0.387) | C(21)-PRO180 (0.324) | |||
| N(3)-GLU186 (0.267) |
| [1] |
Eleftheriou, P.; Geronikaki, A.; Petrou, A. Curr. Top. Med. Chem. 2019, 19, 246.
doi: 10.2174/1568026619666190201152153 pmid: 30714526 |
| [2] |
Kostrzewa, T.; Styszko, J.; Gorska-Ponikowska, M.; Sledzinski, T.; Kuban-Jankowska, A. Anticancer Res. 2019, 39, 3379.
doi: 10.21873/anticanres.13481 pmid: 31262859 |
| [3] |
Kuban-Jankowska, A.; Gorska-Ponikowska, M.; Wozniak, M. Anticancer Res. 2017, 37, 2893.
pmid: 28551626 |
| [4] |
Gulipalli, K. C.; Bodige, S.; Ravula, P.; Endoori, S.; G. R. V.; Babu, G. S.; Chandra, J. N. N. S.; Seelam, N. Bioorg. Med. Chem. Lett. 2017, 27, 3558.
doi: S0960-894X(17)30528-0 pmid: 28579122 |
| [5] |
Lessard, L.; Labbé, D. P.; Deblois, G.; Bégin, L. R.; Hardy, S.; Mes-Masson, A-M.; Saad, F.; Trotman, L. C.; Giguère, V.; Tremblay, M. L. Cancer Res. 2012, 72, 1529.
doi: 10.1158/0008-5472.CAN-11-2602 |
| [6] |
Kostrzewa, T.; Przychodzen, P.; Gorska-Ponikowska, M.; Kuban-Jankowska, A. Anticancer Res. 2019, 39, 745.
doi: 10.21873/anticanres.13171 pmid: 30711953 |
| [7] |
Hussain, H.; Green, I. R.; Abbas, G.; Adekenov, S. M.; Hussain, W.; Ali, I. Expert Opin. Ther. Pat. 2019, 29, 689.
doi: 10.1080/13543776.2019.1655542 |
| [8] |
Guo, S. J.; Wang, L.J, Chen, D.; Jiang, B. RSC Adv. 2020, 10, 3429.
doi: 10.1039/C9RA10660J |
| [9] |
Kim, D. H.; Lee, S.; Chung, Y. W.; Kim, B. M.; Kim, H.; Kim, K.; Yang, K. M. Biomed. Res. Int. 2016, 2016, 8432759.
|
| [10] |
Krishnan, N.; Konidaris, K. F.; Gasser, G.; Tonks, N. K. J. Biol. Chem. 2018, 293, 1517.
doi: 10.1074/jbc.C117.819110 |
| [11] |
Abdelsalam, S. S.; Korashy, H. M.; Zeidan, A.; Agouni, A. Biomolecules 2019, 9, 286.
doi: 10.3390/biom9070286 |
| [12] |
Kumar, A.; Rana, D.; Rana, R.; Bhatia, R. Curr. Mol. Pharm. 2020, 13, 17.
doi: 10.2174/1874467212666190724150723 |
| [13] |
Dodd, G. T.; Xirouchaki, C. E.; Eramo, M.; Mitchell, C. A.; Andrews, Z. B.; Henry, B. A.; Cowley, M. A.; Tiganis, T. Cell Rep. 2019, 28, 2905.
doi: 10.1016/j.celrep.2019.08.019 |
| [14] |
Liu, H. Y.; Sun, D. W.; Du, H.; Zheng, C. J.; Li, J. Y.; Piao, H. R.; Li, J.; Sun, L. P. Eur. J. Med. Chem. 2019, 172, 163.
doi: 10.1016/j.ejmech.2019.03.059 |
| [15] |
Sharma, B.; Xie, L. X.; Yang, F.; Wang, W.; Zhou, Q. M.; Xiang, M. H.; Zhou, S. Z.; Lv, W. T.; Jia, Y.; Pokhrel, L.; Shen, J.; Xiao, Q. C.; Gao, L. Q.; Deng, W. B. Eur. J. Med. Chem. 2020, 199, 112376.
doi: 10.1016/j.ejmech.2020.112376 |
| [16] |
Li, Y.-J.; Liu, X.-J.; Liu, J.-H.; Gao, L.-X.; Jin, K.; Sheng, L.; Yang, H.-J.; Lin, L.-D.; Li, J. Chin. J. Org. Chem. 2020, 40, 478. (in Chinese)
doi: 10.6023/cjoc201907043 |
|
(李英俊, 刘雪洁, 刘季红, 高立信, 靳焜, 盛丽, 杨鸿境, 林乐弟, 李佳, 有机化学, 2020, 40, 478.)
doi: 10.6023/cjoc201907043 |
|
| [17] |
Li, Y.-J.; Yang, H.-J.; Cao, X.; Gao, L.-X.; Jin, K.; Sheng, L.; Liu, J.-H.; Liu, X.-J.; Li, J. Chin. J. Appl. Chem. 2020, 37, 994. (in Chinese)
|
|
(李英俊, 杨鸿境, 曹欣, 高立信, 靳焜, 盛丽, 刘继红, 刘雪洁, 李佳, 应用化学, 2020, 37, 994.)
|
|
| [18] |
Alotabi, S. H. Arabian J. Chem. 2020, 13, 4771.
doi: 10.1016/j.arabjc.2019.12.006 |
| [19] |
Hamoud, M. M. S.; Ghanim, A, M.; Osman, N. A.; Hassan, A. E. A.; Abdel-Fattah, H. A.; Sebaiy, M. M. Med. Analy. Chem. Int. J. 2020, 4, 000159.
|
| [20] |
Liu, K.; Ding, Y. Y.; Kang, C. M. Pharm. Chem. J. 2020, 54, 345.
doi: 10.1007/s11094-020-02215-w |
| [21] |
Aarjane, M.; Aouidate, A.; Slassi, S.; Amine, A. Arabian J. Chem. 2020, 13, 6236.
doi: 10.1016/j.arabjc.2020.05.034 |
| [22] |
Sinha, R.; Singh, U. V. S.; Khosa, R. L.; Jain, J. J. Appl. Pharm. Sci. Res. 2018, 1, 16.
|
| [23] |
Costa, G. C.; Montagnoli, T. L.; Soares Da Silva, J.; Nogueira de Alencar, A. K.; Gamba, L. E. R.; Alves, B. E. O.; Carvalho da Silva, M. M.; Trachez, M. M.; M do Nascimento, J. H.; Pimentel-Coelho, P. M.; Mendez-Otero, R.; Lima, L. M.; Barreiro, E. J.; Sudo, R. T.; Zapata-Sudo, G. Drug Des. Dev. Ther. 2020, 14, 3337.
doi: 10.2147/DDDT.S258459 |
| [24] |
Cordeiro, N. de, M.; Freitas, R. H. C. N.; Fraga, C. A. M.; Fernandes, P. D. J. Pharmacol. Exp. Ther. 2020, 374, 420.
doi: 10.1124/jpet.120.000074 pmid: 32546529 |
| [25] |
Balakrishnan, R.; Vijayraja, D.; Jo, S.-H.; Ganesan, P.; Su-Kim, I.; Choi, D.-K. Antioxid 2020, 9, 101.
doi: 10.3390/antiox9020101 |
| [26] |
Chena, Y. M.; Cao, N. K.; Lv, H. N.; Zeng, K. W.; Yuan, J. Q.; Guo, X. Y.; Zhao, M. B.; Tu, P. F.; Jiang, Y. Phytochemistry 2020, 170, 112186.
doi: 10.1016/j.phytochem.2019.112186 |
| [27] |
Wang, W. B.; Zhou, Z. G.; Zhou, X. H.; Chen, L. M.; Bie, S.; Jing, Z. J. AMB Express 2020, 10, 148.
doi: 10.1186/s13568-020-01074-8 |
| [28] |
Yamashita, S.; Honda, R.; Fukuoka, M.; Kimurab, T.; Hosokawa- Mutoc, J.; Kuwataa, K. Prion 2020, 14, 42.
doi: 10.1080/19336896.2020.1714372 pmid: 31971853 |
| [29] |
Liu, Y. H.; Wu, Y. B.; Sun, L. Q.; Gu, Y. X.; Hu, L. X. Eur. J. Med. Chem. 2020, 191, 112181.
doi: 10.1016/j.ejmech.2020.112181 |
| [30] |
Sadeghian, B.; Sakhteman, A.; Faghih, Z.; Nadri, H.; Edraki, N.; Iraji, A.; Sadeghian, I.; Rezaei, Z. J. Mol. Struct. 2020, 1221, 128793.
doi: 10.1016/j.molstruc.2020.128793 |
| [31] |
Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M. V.; Bruno, R.; Aquaro, S.; Sinicropi, M. S. Molecules 2019, 24, 1912.
doi: 10.3390/molecules24101912 |
| [32] |
Aminah, N. S.; Thant, T. M.; Kristanti, A. N.; Ramadhan, R.; Aung, H. T.; Takaya, Y. Nat. Prod. Commun. 2020, 14, 1.
|
| [33] |
Sullivan, H.-J.; Wang, X. Y.; Nogle, S.; Liao, S.; Wu, C. PPAR Res. 2020, 2020, 5314187.
|
| [34] |
Sutkuvienė, S.; Sakalauskaitė, S.; Kuliešienė, N.; Ragelienė, L.; Daugelavičius, R. Biologija 2020, 66, 80.
|
| [35] |
Mehta, S.; Kumar, S.; Marwaha, R. K.; Narasimhan, B.; Ramasamy, K.; Lim, S. M.; Shah, S. A. A.; Mani, V. BMC Chem. 2019, 13, 113.
doi: 10.1186/s13065-019-0629-0 |
| [36] |
Kerur, S.; Alagawadi, K.; Zhu, H.-L.; Manvi, F. Indian J. Pharm. Educ. Res. 2016, 50, 465.
doi: 10.5530/ijper |
| [37] |
Aly, S. A. J. Radiat. Res. Appl. Sci. 2017, 11, 163.
|
| [38] |
Ren, W. X.; Ren, Y. J.; Wang, S. Eur. J. Med. Chem. 2016, 120, 148.
doi: 10.1016/j.ejmech.2016.05.020 |
| [39] |
Abdel-Aal, M. T.; El-Sayed, W. A.; El-Ashry, E.-S. H. Arch. Pharm. Chem. Life Sci. 2006, 339, 656.
doi: 10.1002/(ISSN)1521-4184 |
| [40] |
Abdel-Aal, M. T.; El-Sayed, W. A.; El-Kosy, S. M.; El-Ashry, E. S. H. Arch. Pharm. Chem. Life Sci. 2008, 341, 307.
doi: 10.1002/(ISSN)1521-4184 |
| [41] |
El-Sayed, W. A.; Fathi, N. M.; Gad, W. A.; El-Ashry, E. S. H. J. Carbohydr. Chem. 2008, 27, 357.
doi: 10.1080/07328300802262778 |
| [42] |
Li, Y.-J.; Wang, S.-Y.; Jin, K.; Gao, L.-X.; Sheng, L.; Zhang, N.; Liu, J.-H.; Li, J. Chin. J. Org. Chem. 2019, 39, 491. (in Chinese)
doi: 10.6023/cjoc201806042 |
|
(李英俊, 王思远, 靳焜, 高立信, 盛丽, 张楠, 刘继红, 李佳, 有机化学, 2019, 39, 491.)
doi: 10.6023/cjoc201806042 |
|
| [43] |
Ning, Y.-C. Structural Identification of Organic Compounds and Organic Spectroscopy, 2 ed., Science Press, Beijing, 2000, p. 53. (in Chinese)
|
|
(宁永成, 有机化合物结构鉴定与有机波谱学, 科学出版社, 北京, 2000, p. 53.)
|
|
| [44] |
Li, Y.-J.; Zhang, Y.-X.; Xu, Y.-T.; Sun, S.-Q.; Wang, Y.; Liu, S.-N. Chin. J. Org. Chem. 2003, 23, 1442. (in Chinese)
|
|
(李英俊, 张颖新, 许永廷, 孙淑琴, 王营, 刘素娜, 有机化学, 2003, 23, 1442.)
|
|
| [45] |
Sun, L. P.; Shen, Q.; Piao, H. H.; Ma, W. P.; Gao, L. X.; Zhang, W.; Nan, F. J.; Li, J.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3630.
doi: 10.1016/j.ejmech.2011.05.027 |
| [1] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [5] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [6] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [7] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [8] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [9] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [10] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [11] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [12] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [13] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [14] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [15] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||