有机化学 ›› 2022, Vol. 42 ›› Issue (2): 607-618.DOI: 10.6023/cjoc202108009 上一篇 下一篇
研究论文
王伟a, 武复冉a, 马一丹a, 徐丹b,c,*( ), 徐功a,c,*(
), 徐功a,c,*( )
)
收稿日期:2021-08-07
修回日期:2021-09-18
发布日期:2022-02-24
通讯作者:
徐丹, 徐功
基金资助:
Wei Wanga, Furan Wua, Yidan Maa, Dan Xub,c( ), Gong Xua,c(
), Gong Xua,c( )
)
Received:2021-08-07
Revised:2021-09-18
Published:2022-02-24
Contact:
Dan Xu, Gong Xu
Supported by:文章分享

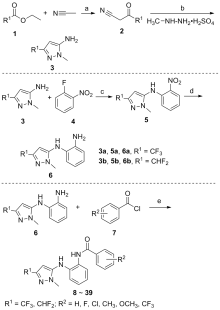
为了找寻新的杀菌剂, 运用活性片段拼接策略, 通过亚胺键将苯甲酰胺类衍生物和吡唑环连接起来, 设计并合成了一系列共计32个新型吡唑-5-基-苯甲酰胺类衍生物, 其结构经过NMR、HRMS分析确认, 并评估了它们的抗真菌活性. 生物测定数据显示, 大多数化合物表现出良好的抑制活性. 4-氯-N-(2-((1-甲基-3-(三氟甲基)-1H-吡唑-5-基)氨基)苯基)苯甲酰胺(14)对油菜菌核病菌和烟草赤星病菌表现出较好的体外活性, EC50值分别为11.21和16.70 mg/L; 对于苹果腐烂病菌, 化合物14 (EC50=11.67 mg/L)活性明显高于阳性对照药剂氟唑菌酰胺(EC50=16.49 mg/L). 分子对接模拟表明, 化合物14通过氢键和p-π作用力与琥珀酸脱氢酶(SDH)的TYR 58和ARG 43相互作用, 这可以解释化合物14与靶蛋白作用的可能机制. 以上结果表明, 化合物14可能是潜在的SDH抑制剂, 并为进一步研究提供有价值的参考.
王伟, 武复冉, 马一丹, 徐丹, 徐功. 含取代吡唑新型苯甲酰胺类化合物的合成及抗真菌活性研究[J]. 有机化学, 2022, 42(2): 607-618.
Wei Wang, Furan Wu, Yidan Ma, Dan Xu, Gong Xu. Study on Synthesis and Antifungal Activity of Novel Benzamides Containing Substituted Pyrazole Unit[J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 607-618.

| 化合物 | R1 | R2 | 油菜菌核病菌 | 苹果腐烂病菌 | 烟草赤星病菌 | 玉米弯孢病菌 | 葡萄灰霉病菌 |
|---|---|---|---|---|---|---|---|
| 8 | CF3 | H | 52.08±1.71 | 50.72±3.17 | 56.57±2.11 | 42.25±1.03 | 38.13±7.11 |
| 9 | CF3 | 2-F | 81.22±3.54 | 64.31±6.88 | 73.68±0.86 | 45.42±1.42 | 58.52±2.44 |
| 10 | CF3 | 3-F | 71.73±1.99 | 62.84±1.41 | 59.21±0.41 | 41.27±1.17 | 52.86±3.33 |
| 11 | CF3 | 4-F | 67.96±5.25 | 60.68±4.58 | 65.45±1.04 | 41.28±2.74 | 47.90±4.21 |
| 12 | CF3 | 2-Cl | 80.70±1.16 | 68.91 ±4.56 | 61.52±0.93 | 38.14±3.43 | 54.97±5.46 |
| 13 | CF3 | 3-Cl | 87.99±1.96 | 82.38±1.30 | 64.47±0.66 | 47.21±0.53 | 68.82±3.08 |
| 14 | CF3 | 4-Cl | 89.72±2.72 | 86.72±0.61 | 66.25±0.73 | 49.60±1.20 | 55.60±2.15 |
| 15 | CF3 | 2-CF3 | 81.86±1.14 | 70.58±1.24 | 59.54±0.13 | 46.44±4.25 | 66.72±3.98 |
| 16 | CF3 | 3-CF3 | 35.08±4.33 | 31.68±5.21 | 20.39±1.38 | 13.35±2.49 | 37.88±2.83 |
| 17 | CF3 | 4-CF3 | 29.35±2.67 | 25.63±6.12 | 17.76±0.57 | 25.88±1.08 | 38.67±6.48 |
| 18 | CF3 | 2-CH3 | 61.85±3.47 | 54.59±5.01 | 48.03±0.28 | 36.23±4.24 | 48.50±1.02 |
| 19 | CF3 | 3-CH3 | 35.24±4.23 | 28.28±3.35 | 21.70±0.91 | 23.53±3.60 | 22.73±6.60 |
| 20 | CF3 | 4-CH3 | 82.80±1.40 | 89.95±0.63 | 58.22±1.45 | 50.38±1.98 | 55.41±2.72 |
| 21 | CF3 | 2-OCH3 | 65.30±2.03 | 54.53±4.30 | 51.98±0.75 | 24.39±1.32 | 36.47±3.42 |
| 22 | CF3 | 3-OCH3 | 64.64±3.29 | 60.27±5.18 | 54.28±0.59 | 39.25±2.33 | 33.06±1.46 |
| 23 | CF3 | 4-OCH3 | 75.43±1.04 | 79.72±1.55 | 55.19±1.53 | 47.05±3.34 | 50.42±3.87 |
| 24 | CHF2 | H | 62.64±3.40 | 32.89±5.39 | 35.85±0.75 | 47.75±3.03 | 33.47±4.14 |
| 25 | CHF2 | 2-F | 41.09±2.11 | 40.10±7.63 | 48.04±1.57 | 37.02±3.65 | 33.66±2.53 |
| 26 | CHF2 | 3-F | 60.73±7.33 | 36.71±6.59 | 39.15±1.27 | 25.37±1.05 | 51.45±8.28 |
| 27 | CHF2 | 4-F | 44.79±0.80 | 44.55±3.40 | 38.82±1.45 | 24.77±0.65 | 48.46±3.99 |
| 28 | CHF2 | 2-Cl | 55.77±1.94 | 56.96±4.78 | 50.98±0.48 | 35.40±1.87 | 72.36±3.68 |
| 29 | CHF2 | 3-Cl | 84.94±0.83 | 72.29±2.17 | 58.22±0.41 | 45.61±0.45 | 70.42±6.84 |
| 30 | CHF2 | 4-Cl | 83.42±0.36 | 73.18±2.35 | 52.31±1.06 | 47.56±0.67 | 51.88±4.87 |
| 31 | CHF2 | 2-CF3 | 49.10±0.68 | 46.60±5.94 | 41.11±0.52 | 32.65±0.33 | 32.28±6.58 |
| 32 | CHF2 | 3-CF3 | 80.02±1.48 | 66.90±3.16 | 56.58±0.14 | 33.15±3.38 | 73.25±3.92 |
| 33 | CHF2 | 4-CF3 | 30.49±4.19 | 31.32±5.49 | 25.00±2.40 | 14.43±1.23 | 63.35±7.24 |
| 34 | CHF2 | 2-CH3 | 43.27±5.48 | 42.28±2.61 | 41.77±1.17 | 32.05±0.54 | 54.95±7.68 |
| 35 | CHF2 | 3-CH3 | 44.09±2.12 | 59.53±2.66 | 46.71±0.91 | 27.71±1.23 | 25.06±0.81 |
| 36 | CHF2 | 4-CH3 | 70.16±1.97 | 55.18±2.34 | 44.74±0.60 | 43.86±2.85 | 28.40±4.13 |
| 37 | CHF2 | 2-OCH3 | 63.44±1.42 | 58.43±1.58 | 56.25±0.73 | 37.29±2.16 | 36.51±3.80 |
| 38 | CHF2 | 3-OCH3 | 63.48±1.50 | 38.35±3.45 | 42.76±0.60 | 44.24±3.42 | 24.84±6.94 |
| 39 | CHF2 | 4-OCH3 | 46.79±4.29 | 44.15±3.47 | 45.73±1.55 | 36.98±3.94 | 25.32±4.76 |
| 5IIcc | CHF2 | 3-CH3 | 100 | 95.00±0.41 | 67.62±2.24 | 52.42±1.34 | 89.33±1.92 |
| 氟唑菌酰胺b | 100 | 81.30±2.70 | 95.07±0.65 | 100 | 63.16±1.24 |
| 化合物 | R1 | R2 | 油菜菌核病菌 | 苹果腐烂病菌 | 烟草赤星病菌 | 玉米弯孢病菌 | 葡萄灰霉病菌 |
|---|---|---|---|---|---|---|---|
| 8 | CF3 | H | 52.08±1.71 | 50.72±3.17 | 56.57±2.11 | 42.25±1.03 | 38.13±7.11 |
| 9 | CF3 | 2-F | 81.22±3.54 | 64.31±6.88 | 73.68±0.86 | 45.42±1.42 | 58.52±2.44 |
| 10 | CF3 | 3-F | 71.73±1.99 | 62.84±1.41 | 59.21±0.41 | 41.27±1.17 | 52.86±3.33 |
| 11 | CF3 | 4-F | 67.96±5.25 | 60.68±4.58 | 65.45±1.04 | 41.28±2.74 | 47.90±4.21 |
| 12 | CF3 | 2-Cl | 80.70±1.16 | 68.91 ±4.56 | 61.52±0.93 | 38.14±3.43 | 54.97±5.46 |
| 13 | CF3 | 3-Cl | 87.99±1.96 | 82.38±1.30 | 64.47±0.66 | 47.21±0.53 | 68.82±3.08 |
| 14 | CF3 | 4-Cl | 89.72±2.72 | 86.72±0.61 | 66.25±0.73 | 49.60±1.20 | 55.60±2.15 |
| 15 | CF3 | 2-CF3 | 81.86±1.14 | 70.58±1.24 | 59.54±0.13 | 46.44±4.25 | 66.72±3.98 |
| 16 | CF3 | 3-CF3 | 35.08±4.33 | 31.68±5.21 | 20.39±1.38 | 13.35±2.49 | 37.88±2.83 |
| 17 | CF3 | 4-CF3 | 29.35±2.67 | 25.63±6.12 | 17.76±0.57 | 25.88±1.08 | 38.67±6.48 |
| 18 | CF3 | 2-CH3 | 61.85±3.47 | 54.59±5.01 | 48.03±0.28 | 36.23±4.24 | 48.50±1.02 |
| 19 | CF3 | 3-CH3 | 35.24±4.23 | 28.28±3.35 | 21.70±0.91 | 23.53±3.60 | 22.73±6.60 |
| 20 | CF3 | 4-CH3 | 82.80±1.40 | 89.95±0.63 | 58.22±1.45 | 50.38±1.98 | 55.41±2.72 |
| 21 | CF3 | 2-OCH3 | 65.30±2.03 | 54.53±4.30 | 51.98±0.75 | 24.39±1.32 | 36.47±3.42 |
| 22 | CF3 | 3-OCH3 | 64.64±3.29 | 60.27±5.18 | 54.28±0.59 | 39.25±2.33 | 33.06±1.46 |
| 23 | CF3 | 4-OCH3 | 75.43±1.04 | 79.72±1.55 | 55.19±1.53 | 47.05±3.34 | 50.42±3.87 |
| 24 | CHF2 | H | 62.64±3.40 | 32.89±5.39 | 35.85±0.75 | 47.75±3.03 | 33.47±4.14 |
| 25 | CHF2 | 2-F | 41.09±2.11 | 40.10±7.63 | 48.04±1.57 | 37.02±3.65 | 33.66±2.53 |
| 26 | CHF2 | 3-F | 60.73±7.33 | 36.71±6.59 | 39.15±1.27 | 25.37±1.05 | 51.45±8.28 |
| 27 | CHF2 | 4-F | 44.79±0.80 | 44.55±3.40 | 38.82±1.45 | 24.77±0.65 | 48.46±3.99 |
| 28 | CHF2 | 2-Cl | 55.77±1.94 | 56.96±4.78 | 50.98±0.48 | 35.40±1.87 | 72.36±3.68 |
| 29 | CHF2 | 3-Cl | 84.94±0.83 | 72.29±2.17 | 58.22±0.41 | 45.61±0.45 | 70.42±6.84 |
| 30 | CHF2 | 4-Cl | 83.42±0.36 | 73.18±2.35 | 52.31±1.06 | 47.56±0.67 | 51.88±4.87 |
| 31 | CHF2 | 2-CF3 | 49.10±0.68 | 46.60±5.94 | 41.11±0.52 | 32.65±0.33 | 32.28±6.58 |
| 32 | CHF2 | 3-CF3 | 80.02±1.48 | 66.90±3.16 | 56.58±0.14 | 33.15±3.38 | 73.25±3.92 |
| 33 | CHF2 | 4-CF3 | 30.49±4.19 | 31.32±5.49 | 25.00±2.40 | 14.43±1.23 | 63.35±7.24 |
| 34 | CHF2 | 2-CH3 | 43.27±5.48 | 42.28±2.61 | 41.77±1.17 | 32.05±0.54 | 54.95±7.68 |
| 35 | CHF2 | 3-CH3 | 44.09±2.12 | 59.53±2.66 | 46.71±0.91 | 27.71±1.23 | 25.06±0.81 |
| 36 | CHF2 | 4-CH3 | 70.16±1.97 | 55.18±2.34 | 44.74±0.60 | 43.86±2.85 | 28.40±4.13 |
| 37 | CHF2 | 2-OCH3 | 63.44±1.42 | 58.43±1.58 | 56.25±0.73 | 37.29±2.16 | 36.51±3.80 |
| 38 | CHF2 | 3-OCH3 | 63.48±1.50 | 38.35±3.45 | 42.76±0.60 | 44.24±3.42 | 24.84±6.94 |
| 39 | CHF2 | 4-OCH3 | 46.79±4.29 | 44.15±3.47 | 45.73±1.55 | 36.98±3.94 | 25.32±4.76 |
| 5IIcc | CHF2 | 3-CH3 | 100 | 95.00±0.41 | 67.62±2.24 | 52.42±1.34 | 89.33±1.92 |
| 氟唑菌酰胺b | 100 | 81.30±2.70 | 95.07±0.65 | 100 | 63.16±1.24 |
| 真菌 | 化合物 | R1 | R2 | EC50/(mg•L–1) | 95%置信区间 | 毒力回归方程 | R2 |
|---|---|---|---|---|---|---|---|
| 油菜菌核病菌 | 9 | CF3 | 2-F | 18.90 | 11.96~37.56 | y=–1.684+1.319x | 0.931 |
| 10 | CF3 | 3-F | 21.58 | 16.37~30.82 | y=–1.289+0.966x | 0.932 | |
| 11 | CF3 | 4-F | 30.11 | 23.06~42.76 | y=–1.687+1.141x | 0.948 | |
| 12 | CF3 | 2-Cl | 20.07 | 12.49~42.19 | y=–1.772+1.360x | 0.929 | |
| 13 | CF3 | 3-Cl | 15.09 | 9.36~28.97 | y=–1.718+1.457x | 0.925 | |
| 14 | CF3 | 4-Cl | 11.21 | 7.32~18.12 | y=–1.613+1.537x | 0.941 | |
| 15 | CF3 | 2-CF3 | 14.74 | 9.43~26.83 | y=–1.360+1.164x | 0.927 | |
| 20 | CF3 | 4-CH3 | 15.99 | 9.72~32.97 | y=–1.492+1.240x | 0.920 | |
| 23 | CF3 | 4-OCH3 | 21.98 | 13.64~47.94 | y=–1.595+1.188x | 0.932 | |
| 29 | CHF2 | 3-Cl | 17.96 | 11.24~35.80 | y=–1.859+1.482x | 0.931 | |
| 30 | CHF2 | 4-Cl | 18.73 | 11.85~37.00 | y=–1.864+1.464x | 0.935 | |
| 32 | CHF2 | 3-CF3 | 19.49 | 11.86~43.08 | y=–1.639+1.271x | 0.921 | |
| 36 | CHF2 | 4-CH3 | 23.76 | 18.25~33.32 | y=–1.451+1.055x | 0.957 | |
| 氟唑菌酰胺a | 0.55 | 0.20~0.98 | y=0.253+0.974x | 0.930 | |||
| 苹果腐烂病菌 | 9 | CF3 | 2-F | 28.46 | 19.88~47.86 | y=–1.150+0.91x | 0.926 |
| 10 | CF3 | 3-F | 27.26 | 18.52~48.47 | y=–1.025+0.714x | 0.915 | |
| 11 | CF3 | 4-F | 28.84 | 19.18~53.99 | y=–0.999+0.684x | 0.958 | |
| 12 | CF3 | 2-Cl | 20.86 | 14.98~32.71 | y=–1.028+0.779x | 0.918 | |
| 13 | CF3 | 3-Cl | 11.17 | 6.91~19.58 | y=–1.249+1.191x | 0.918 | |
| 14 | CF3 | 4-Cl | 11.67 | 7.51~19.69 | y=–1.138+1.067x | 0.933 | |
| 15 | CF3 | 2-CF3 | 19.23 | 14.12~28.78 | y=–1.059+0.829x | 0.928 | |
| 20 | CF3 | 4-CH3 | 16.14 | 9.92~32.04 | y=–1.957+1.620x | 0.922 | |
| 23 | CHF2 | 4-OCH3 | 16.23 | 10.87~28.38 | y=–1.423+1.175x | 0.939 | |
| 29 | CHF2 | 3-Cl | 16.01 | 11.98~22.91 | y=–1.019+0.846x | 0.940 | |
| 30 | CHF2 | 4-Cl | 18.86 | 14.56~25.99 | y=–1.277+1.001x | 0.946 | |
| 32 | CHF2 | 3-CF3 | 20.83 | 16.63~27.36 | y=–1.599+1.212x | 0.961 | |
| 36 | CHF2 | 4-CH3 | 46.72 | 31.92~82.28 | y=–1.528+0.916x | 0.974 | |
| 氟唑菌酰胺a | 16.49 | 11.81~27.49 | y=–1.112+0.914x | 0.984 | |||
| 烟草赤星病菌 | 9 | CF3 | 2-F | 17.19 | 4.89~28.20 | y=–1.627+1.317x | 0.990 |
| 10 | CF3 | 3-F | 39.89 | 26.67~73.75 | y=–1.274+0.796x | 0.944 | |
| 11 | CF3 | 4-F | 26.60 | 15.58~71.12 | y=–1.333+0.936x | 0.906 | |
| 12 | CF3 | 2-Cl | 31.06 | 21.76~51.93 | y=–1.234+0.827x | 0.934 | |
| 13 | CF3 | 3-Cl | 25.89 | 19.82~36.60 | y=–1.514+1.071x | 0.979 | |
| 14 | CF3 | 4-Cl | 16.70 | 13.64~20.91 | y=–1.162+0.858x | 0.959 | |
| 15 | CF3 | 2-CF3 | 32.58 | 25.23~70.83 | y=–1.142+0.755x | 0.963 | |
| 20 | CF3 | 4-CH3 | 34.97 | 3.45~62.57 | y=–1.568+1.016x | 0.946 | |
| 23 | CF3 | 4-OCH3 | 33.02 | 21.90~62.36 | y=–1.088+0.716x | 0.938 | |
| 29 | CHF2 | 3-Cl | 37.16 | 24.82~69.89 | y=–1.383+0.881x | 0.948 | |
| 30 | CHF2 | 4-Cl | 50.71 | 31.21~112.50 | y=–0.984+0.577x | 0.993 | |
| 32 | CHF2 | 3-CF3 | 46.83 | 30.65~90.41 | y=–1.344+0.804x | 0.922 | |
| 氟唑菌酰胺a | 0.68 | 0.23~1.27 | y=0.125+0.773x | 0.941 |
| 真菌 | 化合物 | R1 | R2 | EC50/(mg•L–1) | 95%置信区间 | 毒力回归方程 | R2 |
|---|---|---|---|---|---|---|---|
| 油菜菌核病菌 | 9 | CF3 | 2-F | 18.90 | 11.96~37.56 | y=–1.684+1.319x | 0.931 |
| 10 | CF3 | 3-F | 21.58 | 16.37~30.82 | y=–1.289+0.966x | 0.932 | |
| 11 | CF3 | 4-F | 30.11 | 23.06~42.76 | y=–1.687+1.141x | 0.948 | |
| 12 | CF3 | 2-Cl | 20.07 | 12.49~42.19 | y=–1.772+1.360x | 0.929 | |
| 13 | CF3 | 3-Cl | 15.09 | 9.36~28.97 | y=–1.718+1.457x | 0.925 | |
| 14 | CF3 | 4-Cl | 11.21 | 7.32~18.12 | y=–1.613+1.537x | 0.941 | |
| 15 | CF3 | 2-CF3 | 14.74 | 9.43~26.83 | y=–1.360+1.164x | 0.927 | |
| 20 | CF3 | 4-CH3 | 15.99 | 9.72~32.97 | y=–1.492+1.240x | 0.920 | |
| 23 | CF3 | 4-OCH3 | 21.98 | 13.64~47.94 | y=–1.595+1.188x | 0.932 | |
| 29 | CHF2 | 3-Cl | 17.96 | 11.24~35.80 | y=–1.859+1.482x | 0.931 | |
| 30 | CHF2 | 4-Cl | 18.73 | 11.85~37.00 | y=–1.864+1.464x | 0.935 | |
| 32 | CHF2 | 3-CF3 | 19.49 | 11.86~43.08 | y=–1.639+1.271x | 0.921 | |
| 36 | CHF2 | 4-CH3 | 23.76 | 18.25~33.32 | y=–1.451+1.055x | 0.957 | |
| 氟唑菌酰胺a | 0.55 | 0.20~0.98 | y=0.253+0.974x | 0.930 | |||
| 苹果腐烂病菌 | 9 | CF3 | 2-F | 28.46 | 19.88~47.86 | y=–1.150+0.91x | 0.926 |
| 10 | CF3 | 3-F | 27.26 | 18.52~48.47 | y=–1.025+0.714x | 0.915 | |
| 11 | CF3 | 4-F | 28.84 | 19.18~53.99 | y=–0.999+0.684x | 0.958 | |
| 12 | CF3 | 2-Cl | 20.86 | 14.98~32.71 | y=–1.028+0.779x | 0.918 | |
| 13 | CF3 | 3-Cl | 11.17 | 6.91~19.58 | y=–1.249+1.191x | 0.918 | |
| 14 | CF3 | 4-Cl | 11.67 | 7.51~19.69 | y=–1.138+1.067x | 0.933 | |
| 15 | CF3 | 2-CF3 | 19.23 | 14.12~28.78 | y=–1.059+0.829x | 0.928 | |
| 20 | CF3 | 4-CH3 | 16.14 | 9.92~32.04 | y=–1.957+1.620x | 0.922 | |
| 23 | CHF2 | 4-OCH3 | 16.23 | 10.87~28.38 | y=–1.423+1.175x | 0.939 | |
| 29 | CHF2 | 3-Cl | 16.01 | 11.98~22.91 | y=–1.019+0.846x | 0.940 | |
| 30 | CHF2 | 4-Cl | 18.86 | 14.56~25.99 | y=–1.277+1.001x | 0.946 | |
| 32 | CHF2 | 3-CF3 | 20.83 | 16.63~27.36 | y=–1.599+1.212x | 0.961 | |
| 36 | CHF2 | 4-CH3 | 46.72 | 31.92~82.28 | y=–1.528+0.916x | 0.974 | |
| 氟唑菌酰胺a | 16.49 | 11.81~27.49 | y=–1.112+0.914x | 0.984 | |||
| 烟草赤星病菌 | 9 | CF3 | 2-F | 17.19 | 4.89~28.20 | y=–1.627+1.317x | 0.990 |
| 10 | CF3 | 3-F | 39.89 | 26.67~73.75 | y=–1.274+0.796x | 0.944 | |
| 11 | CF3 | 4-F | 26.60 | 15.58~71.12 | y=–1.333+0.936x | 0.906 | |
| 12 | CF3 | 2-Cl | 31.06 | 21.76~51.93 | y=–1.234+0.827x | 0.934 | |
| 13 | CF3 | 3-Cl | 25.89 | 19.82~36.60 | y=–1.514+1.071x | 0.979 | |
| 14 | CF3 | 4-Cl | 16.70 | 13.64~20.91 | y=–1.162+0.858x | 0.959 | |
| 15 | CF3 | 2-CF3 | 32.58 | 25.23~70.83 | y=–1.142+0.755x | 0.963 | |
| 20 | CF3 | 4-CH3 | 34.97 | 3.45~62.57 | y=–1.568+1.016x | 0.946 | |
| 23 | CF3 | 4-OCH3 | 33.02 | 21.90~62.36 | y=–1.088+0.716x | 0.938 | |
| 29 | CHF2 | 3-Cl | 37.16 | 24.82~69.89 | y=–1.383+0.881x | 0.948 | |
| 30 | CHF2 | 4-Cl | 50.71 | 31.21~112.50 | y=–0.984+0.577x | 0.993 | |
| 32 | CHF2 | 3-CF3 | 46.83 | 30.65~90.41 | y=–1.344+0.804x | 0.922 | |
| 氟唑菌酰胺a | 0.68 | 0.23~1.27 | y=0.125+0.773x | 0.941 |
| [1] |
Liu, Z.; Li, Q. X.; Song, B. J. Agric. Food Chem. 2020, 68, 11039.
doi: 10.1021/acs.jafc.0c02376 |
| [2] |
Fisher, M. C.; Hawkins, N. J.; Sanglard, D.; Gurr, S. J. Science 2018, 360, 739.
doi: 10.1126/science.aap7999 |
| [3] |
Wang, W.; Zhang, S.; Wang, J. H.; Wu, F. R.; Wang, T.; Xu, G. J. Agric. Food Chem. 2021, 69, 491.
doi: 10.1021/acs.jafc.0c06700 |
| [4] |
Patriarca, A. Curr. Opin. Food Sci. 2019, 29, 42.
doi: 10.1016/j.cofs.2019.08.002 |
| [5] |
Wang, W.; Cheng, X.; Cui, X.; Xia, D. G.; Wang, Z. Q.; Lü, X. H. Pest Manage. Sci. 2021, 77, 3529.
doi: 10.1002/ps.v77.7 |
| [6] |
Ishii, H.; Zhen, F.; Hu, M. J.; Li, X. P.; Schnabel, G. Pest Manage. Sci. 2016, 72, 1844.
doi: 10.1002/ps.4216 |
| [7] |
Li, H.; Gao, M. Q.; Chen, Y.; Wang, Y. X.; Yang, G. F. J. Agric. Food Chem. 2020, 68, 14001.
doi: 10.1021/acs.jafc.0c05646 |
| [8] |
Wei, G.; Huang, M.-W.; Wang, W.-J.; Wu, Y.; Mei, S.-F.; Zhou, L.-M.; Mei, L.-C.; Zhu, X.-L.; Yang, G.-F. J. Agric. Food Chem. 2021, 69, 3965.
doi: 10.1021/acs.jafc.0c07322 |
| [9] |
Xiong, L.; Li, H.; Jiang, L.-N.; Ge, J.-M.; Yang, W.-C.; Zhu, X. L.; Yang, G.-F. J. Agric. Food Chem. 2017, 65, 1021.
doi: 10.1021/acs.jafc.6b05134 |
| [10] |
Xiong, L.; Zhu, X.-L.; Gao, H.-W.; Fu, Y.; Hu, S.-Q.; Jiang, L.-N.; Yang, W.-C.; Yang, G.-F. J. Agric. Food Chem. 2016, 64, 4830.
doi: 10.1021/acs.jafc.6b00325 |
| [11] |
Xiong, L.; Shen, Y.-Q.; Jiang, L.-N.; Zhu, X.-L.; Yang, W.-C.; Huang, W.; Yang, G.-F. ACS Symp. Ser. 2015, 1204, 175.
|
| [12] |
Bardas, G. A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G. S. Pest Manage. Sci. 2010, 66, 967.
doi: 10.1002/ps.v66:9 |
| [13] |
Xiong, L.; Zhu, X.-L.; Shen, Y.-Q.; Wishwa, W. K. W. M.; Li, K.; Yang, G.-F. Eur. J. Med. Chem. 2015, 95, 424.
doi: 10.1016/j.ejmech.2015.03.060 pmid: 25841198 |
| [14] |
Guo, X. F.; Zhao, B.; Fan, Z. J.; Yan, D. Y.; Zhang, N. L.; Wu, Q. F.; Yu, B.; Zhou, S.; Kalinina, T. A.; Belskaya, N. P. J. Agric. Food Chem. 2019, 67, 1647.
doi: 10.1021/acs.jafc.8b06935 |
| [15] |
Jones, D. Nat. Rev. Drug Discovery 2010, 9, 751.
doi: 10.1038/nrd3289 |
| [16] |
Wang, W.; Wang, J. H.; Wu, F. R.; Zhou, H.; Xu, D.; Xu, G. J. Agric. Food Chem. 2021, 69, 5746.
doi: 10.1021/acs.jafc.0c08094 |
| [17] |
Katte, T. A.; Reekie, T. A.; Jorgensen, W. T.; Kassiou, M. J. Org. Chem. 2016, 81, 4883.
doi: 10.1021/acs.joc.6b00710 |
| [18] |
Li, A.; Li, Z.; Zhao, Y.; Yao, T.; Zhao, J. Chin. J. Org. Chem. 2020, 40, 2836. (in Chinese)
doi: 10.6023/cjoc202004013 |
|
( 李安邦, 李中珊, 赵洋, 姚停停, 程敬丽, 赵金浩, 有机化学, 2020, 40, 2836.)
doi: 10.6023/cjoc202004013 |
|
| [19] |
Liu, H.; Xia, D. G.; Hu, R.; Wang, W.; Cheng, X.; Wang, A. L.; Zhang, Q.; Lü, X. H. Pestic. Biochem. Physiol. 2020, 163, 271.
doi: 10.1016/j.pestbp.2019.11.024 |
| [20] |
Lü, X. H.; Ren, Z. L.; Liu, P.; Li, B. X.; Li, Q. S.; Chu, M. J.; Cao, H. Q. Pest Manage. Sci. 2017, 73, 1585.
doi: 10.1002/ps.2017.73.issue-8 |
| [21] |
Zhu, X. L.; Xiong, L.; Li, H.; Song, X. Y.; Liu, J. J.; Yang, G. F. ChemMedChem 2014, 9, 1512.
doi: 10.1002/cmdc.v9.7 |
| [1] | 霍海波, 李桂霞, 王世军, 韩春, 师宝君, 李健. 新型γ-咔啉衍生物的合成及其抑菌活性研究[J]. 有机化学, 2024, 44(1): 204-215. |
| [2] | 马虎, 黄丹凤, 王克虎, 唐朵朵, 冯杨, 任园园, 王君娇, 胡雨来. 3-(三氟甲基)吡唑类化合物的合成[J]. 有机化学, 2023, 43(9): 3257-3267. |
| [3] | 雷容超, 兰文捷, 李梦竹, 傅滨. 苯并磺内酰胺联吡唑化合物的简便合成[J]. 有机化学, 2023, 43(7): 2553-2560. |
| [4] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [5] | 左鑫, 许诗诺, 陈忠洋, 鄢剑锋, 袁耀锋. 茂铁类单分子结电子传输性质的研究进展[J]. 有机化学, 2023, 43(7): 2313-2322. |
| [6] | 何金燕, 田富云, 吴青青, 郑月明, 陈玉婷, 许海燕, 金正盛, 詹丽, 程新强, 顾跃玲, 高召兵, 赵桂龙. 基于[3.3.3]螺桨烷的电压门控钙离子通道α2δ亚基配体的合成和生物活性研究[J]. 有机化学, 2023, 43(6): 2226-2238. |
| [7] | 钟玉梅, 邹小颖, 卓小丫, 王逸涵, 申佳奕, 郑绿茵, 郭维. 4-氧代-2-亚胺基噻唑烷-5-亚基乙酸乙酯类化合物的设计、合成及抗癌活性[J]. 有机化学, 2023, 43(4): 1452-1461. |
| [8] | 刘兴周, 于明加, 梁建华. 原小檗碱骨架的合成及其抗炎活性研究进展[J]. 有机化学, 2023, 43(4): 1325-1340. |
| [9] | 孙洋, 王杨, 张紫婵, 钱烨, 骆桂成, 周贝贝, 缪丽沙, 陈雨蝶, 戴红, 徐宝琳, 吴正光. 新型含1,3,4-噁二唑基团的吡唑肟衍生物的合成与生物活性[J]. 有机化学, 2023, 43(4): 1584-1590. |
| [10] | 张紫婵, 孙洋, 华晟, 徐宝琳, 张敏, 赵勤, 郑丹丹, 王杨, 鞠剑峰, 石玉军, 戴红. 新型含异噁唑单元的吡唑酰胺类衍生物的合成及杀虫活性[J]. 有机化学, 2023, 43(4): 1435-1443. |
| [11] | 李猛, 夏东国, 王云霄, 程祥, 巩杰秀, 陈耀, 吕献海. 苯甲酸噻唑酯类衍生物的设计、合成及抗真菌活性评价[J]. 有机化学, 2023, 43(2): 686-696. |
| [12] | 张雨杉, 桓臻, 杨金东, 程津培. 氮杂环磷氢试剂的氢转移活性研究进展[J]. 有机化学, 2023, 43(11): 3806-3825. |
| [13] | 桑田, 贾帆, 何静, 李春天, 刘岩, 刘平. I2催化β-酮腈与1H-吡唑-5-胺的环化反应[J]. 有机化学, 2023, 43(1): 195-201. |
| [14] | 张蓉, 郜祥, 陈玲玲, 南发俊. 噻唑-噁唑串联杂环类RNA剪接抑制剂的发现及构效关系研究[J]. 有机化学, 2022, 42(9): 2925-2939. |
| [15] | 陈伟, 雷思敏, 兰雨欣, 许豪键, 余坪槟, 张锐, 吴润, 陈阳. 新型喹唑啉酮衍生物的设计合成与抗植物病原真菌活性研究[J]. 有机化学, 2022, 42(7): 2164-2171. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||