有机化学 ›› 2022, Vol. 42 ›› Issue (4): 1123-1128.DOI: 10.6023/cjoc202112007 上一篇 下一篇
研究论文
韩群, 徐坤*( ), 田发宁, 黄胜阳*(
), 田发宁, 黄胜阳*( ), 曾程初*(
), 曾程初*( )
)
收稿日期:2021-12-03
修回日期:2021-12-30
发布日期:2022-01-11
通讯作者:
徐坤, 黄胜阳, 曾程初
基金资助:
Qun Han, Kun Xu( ), Faning Tian, Shengyang Huang(
), Faning Tian, Shengyang Huang( ), Chengchu Zeng(
), Chengchu Zeng( )
)
Received:2021-12-03
Revised:2021-12-30
Published:2022-01-11
Contact:
Kun Xu, Shengyang Huang, Chengchu Zeng
Supported by:文章分享
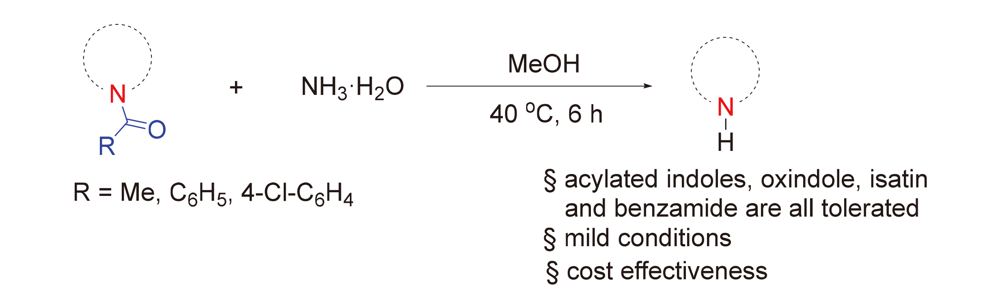
酰胺化合物中的酰基脱除是有机合成化学的重要研究方向之一. 但是, 由于酰胺键较为惰性, 在温和条件下脱除酰基保护基的报道较少. 为了解决上述问题, 发展了一种使用氨水脱除酰基保护基的新方法. 该方法不但条件温和, 而且可以放大规模反应(10 mmol). 一系列药物分子或者药物分子衍生物, 例如吲哚美辛、N-乙酰基褪黑素及N-乙酰基卡布洛芬中的酰基都可以采用此方法高产率脱除. 该方法具有较好的官能团容忍性、操作简便、条件温和、产率高以及所用的试剂廉价易得等优势. 因此, 该方法有望成为N-酰基脱保护的实用方法之一.
韩群, 徐坤, 田发宁, 黄胜阳, 曾程初. 一种利用酰胺基转移反应脱除酰基保护基的实用方法[J]. 有机化学, 2022, 42(4): 1123-1128.
Qun Han, Kun Xu, Faning Tian, Shengyang Huang, Chengchu Zeng. A Practical Transamidation Strategy for the N-Deacylation of Amides[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1123-1128.
| Entry | Deviation from standard conditions | Yield/% |
|---|---|---|
| 1 | None | 91 |
| 2 | r.t. | 68 |
| 3 | 60 ℃ | 90 |
| 4 | DCM instead of MeOH | Trace |
| 5 | DCE instead of MeOH | Trace |
| 6 | THF instead of MeOH | Trace |
| 7 | MeCN instead of MeOH | 47 |
| 8 | EtOH instead of MeOH | 67 |
| 9 | 1 mL MeOH | 56 |
| 10 | 0.8 mL NH3•H2O | 77 |
| 11 | CH3NH2 instead of NH3•H2O | 63 |
| Entry | Deviation from standard conditions | Yield/% |
|---|---|---|
| 1 | None | 91 |
| 2 | r.t. | 68 |
| 3 | 60 ℃ | 90 |
| 4 | DCM instead of MeOH | Trace |
| 5 | DCE instead of MeOH | Trace |
| 6 | THF instead of MeOH | Trace |
| 7 | MeCN instead of MeOH | 47 |
| 8 | EtOH instead of MeOH | 67 |
| 9 | 1 mL MeOH | 56 |
| 10 | 0.8 mL NH3•H2O | 77 |
| 11 | CH3NH2 instead of NH3•H2O | 63 |
| [1] |
(a) Wang, H.; Shi, J.; Tan, J.; Xu, W.; Zhang, S.; Xu, K. Org. Lett. 2019, 21, 9430.
doi: 10.1021/acs.orglett.9b03641 |
|
(b) Wang, X.; Xu, X.; Wang, Z.; Fang, P.; Mei, T. Chin. J. Org. Chem. 2020, 40, 3738. (in Chinese)
doi: 10.6023/cjoc202003022 |
|
|
( 王向阳, 徐学涛, 王振华, 方萍, 梅天胜, 有机化学, 2020, 40, 3738.)
doi: 10.6023/cjoc202003022 |
|
|
(c) Yang, Q.; Yan, X.-T.; Feng, C.-T.; Chen, D.-X.; Yan, Z.-Z.; Xu, K. Org. Chem. Front. 2021, 8, 6384.
doi: 10.1039/D1QO01060C |
|
|
(d) Ge, Y.-X.; Yan, Q.-Q.; Tian, Y.-F.; Wang, H.-J.; Zhang, C.-F.; Li, Z.-J. Chin. J. Org. Chem. 2021, 41, 3106. (in Chinese)
doi: 10.6023/cjoc202102035 |
|
|
( 葛雅欣, 闫芹芹, 田云飞, 王海军, 张春芳, 李泽江, 有机化学, 2021, 41, 3106.)
doi: 10.6023/cjoc202102035 |
|
|
(e) Yi, R.-N.; He, W.-M. Chin. J. Org. Chem. 2021, 41, 1267. (in Chinese)
doi: 10.6023/cjoc202100022 |
|
|
( 易荣楠, 何卫民, 有机化学, 2021, 41, 1267.)
doi: 10.6023/cjoc202100022 |
|
| [2] |
Baran, P. S.; Corey, E. J. J. Am. Chem. Soc. 2002, 124, 7904.
pmid: 12095326 |
| [3] |
(a) Wen, J.; Wang, F.; Zhang, X. M. Chem. Soc. Rev. 2021, 50, 3211.
doi: 10.1039/D0CS00082E |
|
(b) Jiang, X.-L.; Zhu, S.-L. Chin. J. Org. Chem. 2021, 41, 3745. (in Chinese)
doi: 10.6023/cjoc202100068 |
|
|
( 江晓莉, 朱少林, 有机化学, 2021, 41, 3745.)
doi: 10.6023/cjoc202100068 |
|
| [4] |
(a) Zhang, M.; Zhang, Y.; Jie, X.; Zhao, H.; Li, G.; Su, W. P. Org. Chem. Front. 2014, 1, 843.
doi: 10.1039/C4QO00068D |
|
(b) Luo, H.; Pei, N.; Zhang, J. Chin. J. Org. Chem. 2021, 41, 2990. (in Chinese)
doi: 10.6023/cjoc202103013 |
|
|
( 罗欢欢, 裴娜, 张敬, 有机化学, 2021, 41, 2990.)
doi: 10.6023/cjoc202103013 |
|
|
(c) Feng, Y.-L.; Shi, B. Chin. J. Org. Chem. 2021, 41, 3753. (in Chinese)
doi: 10.6023/cjoc202104004 |
|
|
( 冯亚岚, 史炳锋, 有机化学, 2021, 41, 3753.)
|
|
|
(d) Sun, S.-Z.; Wang, X.; Cheng, T.-J.; Xu, H.; Dai, H.-X. Chin. J. Org. Chem. 2020, 40, 3371 (in Chinese)
doi: 10.6023/cjoc202005064 |
|
|
( 孙尚政, 王星, 程泰锦, 徐辉, 戴辉雄, 有机化学, 2020, 40, 3371.)
doi: 10.6023/cjoc202005064 |
|
|
(e) Liu, Y.-Y.; Zhang, Y.; Wan, J.-P. J. Org. Chem. 2017, 82, 8950.
doi: 10.1021/acs.joc.7b01375 |
|
|
(f) Du, Y.; Liu, Y.-Y.; Wan, J.-P. J. Org. Chem. 2018, 83, 3403.
doi: 10.1021/acs.joc.8b00068 |
|
| [5] |
(a) Wuts, P. G. M.; Greene, T. W. Protective Groups in Organic Synthesis, 4th ed., Wiley-Interscience, Hoboken, 2006, pp. 773-789.
|
|
(b) Yoo, M.; Jung, K.-W. ChemistrySelect 2018, 3, 1527.
doi: 10.1002/slct.201702289 |
|
| [6] |
Spaggiari, A.; Blaszczak, L. C.; Prati, F. Org. Lett. 2004, 6, 3885.
pmid: 15496055 |
| [7] |
Koenig, S. G.; Vandenbossche, C. P.; Zhao, H.; Mousaw, P.; Singh, S. P.; Bakale, R. P. Org. Lett. 2009, 11, 433.
doi: 10.1021/ol802482d |
| [8] |
Sultane, P. R.; Mete, T. B.; Bhat, R. G. Org. Biomol. Chem. 2014, 12, 261.
doi: 10.1039/C3OB41971A |
| [9] |
Wang, A.-E.; Chang, Z.; Liu, Y.-P.; Huang, P.-Q. Chin. Chem. Lett. 2015, 26, 1055.
doi: 10.1016/j.cclet.2015.05.033 |
| [10] |
Kita, Y.; Nishii, Y.; Onoue, A.; Mashima, K. Adv. Synth. Catal. 2013, 355, 3391.
doi: 10.1002/adsc.201300819 |
| [11] |
Shimizu, Y.; Morimoto, H.; Zhang, M.; Ohshima, T. Angew. Chem., Int. Ed. 2012, 51, 8564.
doi: 10.1002/anie.201202354 |
| [12] |
(a) Lian, F.; Xu, K. Chin. J. Org. Chem. 2020, 40, 3490. (in Chinese)
doi: 10.6023/cjoc202000072 |
|
( 廉菲, 徐坤, 有机化学, 2020, 40, 3490.)
doi: 10.6023/cjoc202000072 |
|
|
(b) Meng, Z.-Y.; Feng, C.-T.; Xu, K. Chin. J. Org. Chem. 2021, 41, 2535. (in Chinese)
doi: 10.6023/cjoc202012013 |
|
|
( 蒙泽银, 冯承涛, 徐坤, 有机化学, 2021, 41, 2535.)
doi: 10.6023/cjoc202012013 |
|
|
(c) Lian, F.; Xu, K.; Zeng, C. Chem. Rec. 2021, 21, 2290.
doi: 10.1002/tcr.202100036 |
|
|
(d) Li, J.; Zhang, S.; Xu, K. Chin. Chem. Lett. 2021, 32, 2729.
doi: 10.1016/j.cclet.2021.03.027 |
|
|
(e) Zhang, S.; Li, L.; Li, J.; Shi, J.; Xu, K.; Gao, W.; Zong, L.; Li, G.; Findlater, M. Angew. Chem., Int. Ed. 2021, 60, 7275.
doi: 10.1002/anie.202015230 |
|
|
(f) Jiang, Y.; Xu, K.; Zeng, C. CCS Chem. 2021, 3, 1911.
|
|
| [13] |
Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Curr. Neuropharmacol. 2017, 15, 434.
doi: 10.2174/1570159X14666161228122115 pmid: 28503116 |
| [14] |
Wang, Y.-H.; Tian, J.-S.; Tan, P.-W.; Cao, Q.; Zhang, X.-X.; Cao, Z.-Y.; Zhou, F.; Wang, X.; Zhou, J. Angew Chem., Int. Ed. 2020, 59, 1634.
doi: 10.1002/anie.201910864 |
| [15] |
Kerr, W. J.; Lindsay, D. M.; Owens, P. K.; Reid, M.; Tuttle, T.; Campos, S. ACS Catal. 2017, 7, 7182.
doi: 10.1021/acscatal.7b02682 |
| [16] |
Roth, G. J.; Heckel, A.; Colbatzky, F.; Handschuh, S.; Kley, J.; Lintz, T. L.; Lotz, R.; Grunt, U. T.; Walter, R.; Hilberg, F. J. Med. Chem. 2009, 52, 4466.
doi: 10.1021/jm900431g |
| [17] |
Castelo-Branco, F. S.; Lima, E. C.; Domingos, J. L. O.; Pintob, A. C.; Lourenço, M. C. S.; Gomes, K. M.; Costa-Lima, M. M.; Araujo-Lima, C. F.; Aiub, C. A. F.; Felzenszwalb, I.; Costa, T. E. M. M.; Penido, C.; Henriques, M. G.; Boechat, N. Eur. J. Med. Chem. 2018, 146, 529.
doi: S0223-5234(18)30084-9 pmid: 29407978 |
| [18] |
He, K.-H.; Tan, F.-F.; Zhou, C.-Z.; Zhou, G.-J.; Yang, X.-L.; Li, Y. Angew. Chem., Int. Ed. 2017, 56, 3080.
doi: 10.1002/anie.201612486 |
| [19] |
Wang, Q.-F.; Chai, H.; Yu, Z.-K. Organometallics 2018, 37, 584.
doi: 10.1021/acs.organomet.7b00902 |
| [20] |
Wu, J.-J.; Talwar, D.; Johnston, S.; Yan, M.; Xiao, J.-L. Angew. Chem., Int. Ed. 2013, 52, 6983.
|
| [21] |
Huang, Y.-Q.; Song, H.-J.; Liu, Y.-X.; Wang, Q.-M. Chem.-Eur. J. 2018, 24, 2065.
doi: 10.1002/chem.201705202 |
| [22] |
Zhang, J.-Y.; Chen, S.-Y.; Chen, F.-F.; Xu, W.-S.; Deng, G.-J.; Gong, H. Adv. Synth. Catal. 2017, 359, 2358.
doi: 10.1002/adsc.201700178 |
| [23] |
Ning, X.-S.; Liang, X.; Hu, K.-F.; Yao, C.-Z.; Qu, J.-P.; Kang, Y.-B. Adv. Synth. Catal. 2018, 360, 1590.
doi: 10.1002/adsc.201701512 |
| [24] |
Barykina, O. V.; Snider, B. B. Org. Lett. 2010, 12, 2664.
doi: 10.1021/ol100896n pmid: 20446668 |
| [25] |
Song, W. Z.; Dong, K.; Li, M. Org. Lett. 2020, 22, 371.
doi: 10.1021/acs.orglett.9b03905 |
| [26] |
Wang, M.; Fan, Q.-L.; Jiang, X.-F. Org. Lett. 2018, 20, 216.
doi: 10.1021/acs.orglett.7b03564 |
| [27] |
Omer, H. M.; Liu, P.; Brummond, K. M. J. Org. Chem. 2020, 85, 7959.
doi: 10.1021/acs.joc.0c00788 |
| [28] |
Lin, W.; Hu, M.-H.; Feng, X.; Fu, L.; Cao, C.-P.; Huang, Z.-B.; Shi, D.-Q. Tetrahedron Lett. 2014, 55, 2238.
doi: 10.1016/j.tetlet.2014.02.072 |
| [29] |
Laursen, S. R.; Jensen, M. T.; Lindhardt, A. T.; Jacobsen, M. F.; Skrydstrup, T. Eur. J. Org. Chem. 2016, 2016, 1881.
doi: 10.1002/ejoc.201600143 |
| [30] |
Zhang, F.; Li, L.; Zhang, J. Sci. Rep. 2019, 9, 2787.
doi: 10.1038/s41598-019-39240-z pmid: 30808919 |
| [1] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [4] | 陶苏艳, 项紫欣, 白俊杰, 万潇, 万小兵. 亚硝酸叔丁酯参与的酰胺水解反应[J]. 有机化学, 2024, 44(2): 550-560. |
| [5] | 江港钟, 林嘉欣, 鲍晓光, 万小兵. 亚硝酸异戊酯活化伯磺酰胺制备磺酰溴与磺酰氯[J]. 有机化学, 2024, 44(2): 533-549. |
| [6] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [7] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [8] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [9] | 黄志友, 杨平, 何波, 欧文霞, 袁思雨. 吗啉磺酰胺化合物的设计、合成及其抑制大豆萌芽活性的研究[J]. 有机化学, 2024, 44(1): 309-315. |
| [10] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| [11] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [12] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [13] | 张俊杰, 徐学涛. (S)-(–)-Xylopinine和(S)-(+)-Laudanosine的不对称合成[J]. 有机化学, 2023, 43(9): 3297-3303. |
| [14] | 雷容超, 兰文捷, 李梦竹, 傅滨. 苯并磺内酰胺联吡唑化合物的简便合成[J]. 有机化学, 2023, 43(7): 2553-2560. |
| [15] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||