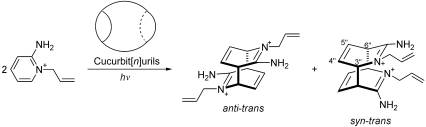
| [1] Everitt S R L, Inoue Y, Ramamurthy V. Org. Photochem.: Mol. Upramo. Photochem, 1999, 3:1[2] Papaefstathiou G S, Kipp A J, MacGillivray L R. Chem.Commun, 2001: 2462[3] Freeman W A, Mock W L, Shih N Y. J. Am. Chem. Soc,1981, 103(56):7367[4] Cong H, Tao Z, Xue S F. Curr. Org. Chem, 2010, In press[5] Mock W L, Shih N Y. J. Org. Chem, 1983, 48:3618 [6] Mock W L, Pierpont J. J. Chem. Soc, Chem. Commun, 1990, 15(9):15[7] Day A I, Arnold A P, Blanck R J. Molecules, 2003, 8(1):74[8] Kim H J, Heo J, Jeon W S, Lee E S, Kim J, Sakamoto S, Yamaguchi K, Kim K. Angew.Chem. Intern. Ed, 2001, 40(8):1526[9] Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L Angew.Chem. Intern. Ed, 2005, 44 (31): 4844 [10] Sokolov M N, Dybtsev D N, Fedin V P. Russ. Chem. Bull., 2003, 52(5):1041[11] Cong H, Zhu Q J, Xue S F, Tao Z, Wei G. Chin. Sci. Bull., 2010, In press [12] 肖昕, 张云黔, 薛赛凤, 祝黔江, 陶朱. 无机化学学报(Xiao X, Zhang Y Q,Xue S F,Zhu Q J, Tao Z. Chin. J. Inorg. Chem), 2009,25:596[13] Buschmann H J, Cleve E, Jansen K.J. Incl. Phen. Macroc., 2000, 1:117[14] 牟兰, 薛赛凤, 祝黔江, 陶朱, 曾晞. 高等学校化学学报(Mou L, Xue S F, Zhu Q J, Tao Z, Zeng X. Chem. J. Chin. Univ)., 2005, 26:1835[15] Mock W L, Irra T A, Wepsiec J P, Manimaran T L. J.Org. Chem ,1983, 48:3619[16] Wang R B, Yuan L, Macartney D H. J. Org. Chem ,2006, 71:1237[17] Jon S Y, Ko Y H, Park S H, Kim H J, Kim K. Chem.Commun , 2001, 1938[18] Mock W L., Irra T A., Wepsiec J P, Adhya M. J. Org. Chem ,1989, 54:5302[19] Mock W L. Top. Curr. Chem, 1995, 175:1[20] Carlqvist P, Maseras F . Chem. Commun., 2007:748 |