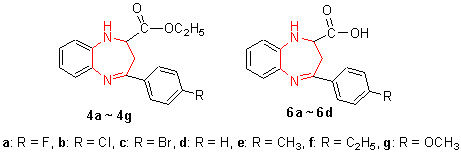
| [1] Mario,D.B.; Giancarlo,G.; Giorgio,R.; Laura,V.; Massimo,M.; Maria,E.M.Eur.J.Med.Chem.2001,36,935.[2] Paolo,F.; Marcello,S.; Mario,D.B.; Luisa,D.; Nicola,P.; Al-fredo,C.; Giancarlo,G.; Marla,R.; Tiziana,M.; Giorgio,R.Pharmacol.Res.2001,43,5.[3] Sang,K.H.; Donthabhaktuni,S.; Eunjung,M.; Murugulla,A.C.; Kagga,M.; Sung-Hoon,K.; Kwang-Hyun,A.; Sun,Y.K.Bio-org.Med.Chem.Lett.2010,20,3969.[4] Liu,J.B.; Lin,C.L.; Wan,Y.Q.; Song,H.C.Chin.J.Org.Chem.2008,28,317 (in Chinese).(刘进兵,林崇懒,万一千,宋化灿,有机化学,2008,28,317.)[5] Konda,S.G.; Shaikh,B.M.; Chavan,S.A.; Dawane,B.S.Chin.Chem.Lett.2011,22,65.[6] Kapil,A.; Anshu,D.Bioorg.Med.Chem.2008,18,114.[7] Sofie,V.D.; Patrick,B.J.Mol.Struct.2010,943,83.[8] Zhao,W.B.; Peng,W.C.; Zhao,N.; Xiao,F.K.; Wei,W.; Sun,Y.H.Petrochem.Technol.2009,38,267 (in Chinese).(赵文波,彭伟才,赵宁,肖福魁,魏伟,孙予罕,石油化工,2009,38,267.)[9] Shu,K.; Tsuyoshi,B.; Satoshi,N.Chem.Eur.J.2006,6,3491.[10] Mehmet,S.K.; Çiğdem, Y.; Mehmet,Y.; Nurcan,K.; Zharmukhamedov,S.K.; Shitov,A.; Los,D.A.; Klimov,V.V.; Allakhverdiev,S.I.Biochim.Biophys.Acta 2012,8,5.[11] Zhu,M.J.; Ge,F.; Zhu,R.L.; Wang,X.Y.; Zheng,X.Y.Chemosphere 2010,80,46.[12] Becke,A.D.J.Chem.Phys.1993,98,5648.[13] Lee,C.; Yang,W.; Parr,R.G.Phys.Rev.B 1988,37,785. [14] Feng,C.J.Acta Chim.Sinica 2012,70,512 (in Chinese).(冯长君,化学学报,2012,70,512.)[15] Yue,K.F.; Zhuo,F.; Hou,L.; Jiang,Y.M.; Zhai,G.H.; Yin,B.; Wang,R.Y.; Wen,Z.Y.Acta Chim.Sinica 2011,69,596 (in Chinese).(岳可芬,卓飞,侯磊,姜严梅,翟高红,尹兵,王尧宇,文振翼,化学学报,2011,69,596.)[16] Long,J.Y.; Yi,H.B.; Liu,X.K.; Wang,Y.F.Acta Chim.Sinica 2012,70,949 (in Chinese).(龙杰义,易海波,刘星楷,汪易非,化学学报,2012,70,949.)[17] Song,X.M.; Chen,F.S.; Wang,S.L.; Liu,F.S.; Wu,H.; Xu,H.W.Acta Chim.Sinica 2011,69,2796 (in Chinese).(宋晓明,陈夫山,王松林,刘福胜,吴海,徐环斐,化学学报,2011,69,2796.)[18] Hu,L.J.M.S.Thesis,Hebei Normal University,Shijiazhuang,2012 (in Chinese).(胡丽娟,硕士论文,河北师范大学,石家庄,2012.) [19] Liang,R.L.; Yuan,J.W.; Qu,L.B.Chin.J.Chin.Med.2011,26,262 (in Chinese).(梁瑞玲,袁金伟,屈凌波,中医学报,2011,26,262.)[20] Xia,S.W.; Sun,W.; Yu,L.M.; Hua,Z.Acta Chim.Sinica 2007,65,2707 (in Chinese).(夏树伟,孙玮,于良民,华哲,化学学报,2007,65,2707.)[21] Huo,N.Organic Synthesis,Science Press,Beijing,1981,pp.67~69 (in Chinese).(E.C霍宁,有机合成,科学出版社,北京,1981,pp.67~69.) |