有机化学 ›› 2019, Vol. 39 ›› Issue (10): 2829-2834.DOI: 10.6023/cjoc201903053 上一篇 下一篇
所属专题: 荧光探针-生物传感合辑; 有机超分子化学合辑
研究论文
朱文博, 朱伟, 丁金东, 马小强, 姚红, 张有明, 林奇*( ), 魏太保*(
), 魏太保*( )
)
收稿日期:2019-03-24
修回日期:2019-05-13
发布日期:2019-06-06
通讯作者:
林奇,魏太保
E-mail:linqi2004@126.com;weitaibao@126.com
基金资助:
Zhu Wenbo, Zhu Wei, Ding Jindong, Ma Xiaoqiang, Yao Hong, Zhang Youming, Lin Qi*( ), Wei Taibao*(
), Wei Taibao*( )
)
Received:2019-03-24
Revised:2019-05-13
Published:2019-06-06
Contact:
Lin Qi,Wei Taibao
E-mail:linqi2004@126.com;weitaibao@126.com
Supported by:文章分享

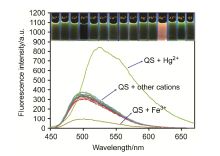
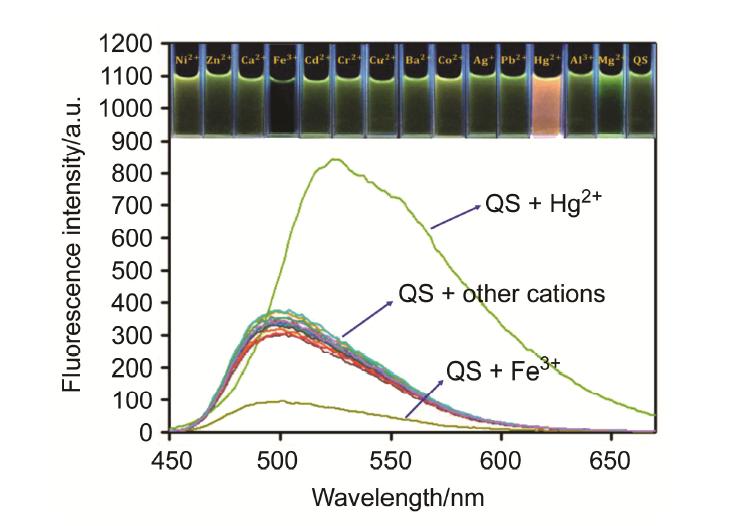
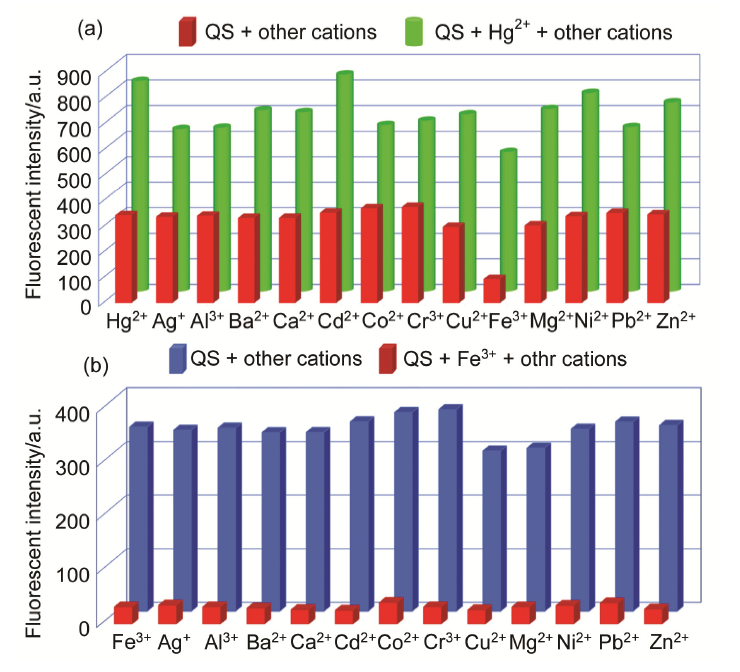
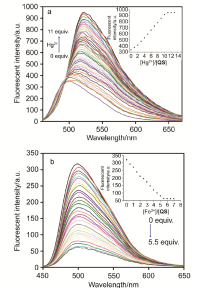
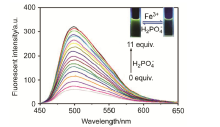
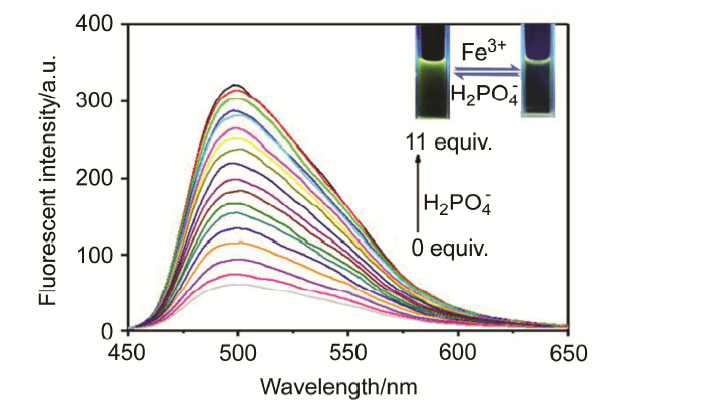
合成了一种新型的基于5-(3-硝基苯基)-呋喃-2-甲醛功能化的巴比妥酸衍生物传感器QS. 通过 1H NMR, 13C NMR, MS等方法对QS进行了表征. QS的荧光光谱结果表明QS的二甲亚砜(DMSO)溶液在498 nm 有最大荧光发射, 在365 nm紫外灯下照射发出绿色荧光. QS对Hg 2+和Fe 3+的水溶液有不同的荧光检测能力, Hg 2+能使探针QS的荧光增强为橙色, Fe 3+使其荧光猝灭, 实现了实时检测. 传感器QS对Hg 2+和Fe 3+的荧光检测限分别为3.25×10 –8和4.0× 10 –8 mol?L –1. 根据Job曲线和质谱研究得到QS与汞离子和铁离子化学计量比均为1∶1, 提出了QS与Hg 2+和Fe 3+的可能结合模式. 加入$H_{2}PO_{4}^{-}$能使含有Fe 3+的QS溶液(QS-Fe)荧光恢复, 可用于Fe 3+的循环检测. 此外, 固体QS可从水溶液中吸附 Hg 2+和Fe 3+(吸附率分别为92.0%和91.8%), 具有较好的吸附能力.
朱文博, 朱伟, 丁金东, 马小强, 姚红, 张有明, 林奇, 魏太保. 基于芳基糠醛功能化巴比妥酸衍生物识别和分离Hg 2+/Fe 3+的新型荧光传感器[J]. 有机化学, 2019, 39(10): 2829-2834.
Zhu Wenbo, Zhu Wei, Ding Jindong, Ma Xiaoqiang, Yao Hong, Zhang Youming, Lin Qi, Wei Taibao. A Novel Fluorescent Sensor Based on Aryl-furfural Functionalized Barbituric Acid for Recognition and Separation of Hg 2+/Fe 3+[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2829-2834.

| [1] | Wang, J.; Li, Y.; Patel, N. G.; Zhang, G.; Zhou, D.; Pang, Y. Chem. Commun. 2014, 50, 12258. |
| [2] | Zhuang, P.; McBride, M. B.; Xia, H.; Li, N.; Li, Z. Sci. Total Environ. 2009, 407, 1551. |
| [3] | Yan, F.; Zou, Y.; Wang, M.; Mu, X.; Yang, N; Chen, L. . Sens. Actuators, B 2014, 192, 488. |
| [4] | Wu, X. F.; Ma, Q. J.; Wei, X. J.; Hou, Y. M.; Zhu, X. Sens. Actuators, B 2013, 183, 565. |
| [5] | Yue, Q.; Shen, T.; Wang, J.; Wang, L.; Xu, S.; Li, H.; Liu, J. Chem. Commun. 2013, 49, 1750. |
| [6] | Kim, H. N.; Ren, W. X.; Kim, J. S.; Yoon, J.S. Chem. Soc. Rev. 2012, 41, 3210. |
| [7] | López-García, I.; Rivas, R. E.; Hernández-Córdoba, M. Anal. Chim. Acta 2012, 743, 69. |
| [8] | Sun, Z.; Du, J.; Jing, C. J. Environ. Sci. 2016, 39, 134. |
| [9] | Yan, Z.; Hu, L.; You, J. Anal. Methods 2016, 8, 5738. |
| [10] | Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. Chem. Soc. Rev. 2011, 40, 3483. |
| [11] | Sahoo, S. K.; Sharma, D.; Bera, R. K.; Crisponic, G.; Callanm, J.F. Chem. Soc. Rev. 2012, 41, 7195. |
| [12] | Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Liu, D.; Chen, H. ACS Appl. Mater. Interfaces 2017, 9, 12663. |
| [13] | Rauchfus, T. B . Acc. Chem. Res. 2015, 48, 2107. |
| [14] |
Zhang, S.; Li, J.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Nanoscale 2014, 6, 4157.
doi: 10.1039/c3nr06744k |
| [15] | Shi, B.; Su, Y.; Zhang, L.; Huang, M.; Liu, R; Zhao, S. . ACS Appl. Mater. Interfaces 2016, 8, 10717. |
| [16] | Han, Y.; Wu, X.; Zhang, X.; Zhou, Z.; Lu, C. ACS Sustainable Chem. Eng. 2016, 4, 5667. |
| [17] |
Khadgi, N.; Upretia, A. R.; Li, Y. RSC Adv. 2017, 7, 27007.
doi: 10.1039/C7RA01782K |
| [18] | Zhang, Y.-Y.; Chen, X.-Z.; Liu, X.-Y.; Wang, M.; Liu, J.-J.; Gao, G.; Zhang, X.-Y.; Sun, R.-Z.; Hou, S.-C.; Wang, H.-M . Sens. Actuators, B. 2018, 273, 1077. |
| [19] |
Sakunkaewkasem, S.; Petdum, A.; Panchan, W.; Sirirak, J.; Charoenpanich, A.; Sooksimuang, T.; Wanichacheva, N. ACS Sens. 2018, 3, 1016.
doi: 10.1021/acssensors.8b00158 |
| [20] | Xia, Q.; Chen, Z.; Zhang, Z.; Liu, R. Chin. J. Org. Chem. 2018, 38, 2700 (in Chinese). |
| ( 夏琦, 陈子康, 张志德, 刘瑞源, 有机化学, 2018, 38, 2700.) | |
| [21] | Wu, Y.-C.; Jiang, K.; Luo, S.-H.; Cao, L.; Wu, H.-Q.; Wang, Z.-Y. Spectrochim. Acta, Part A 2019, 206, 632. |
| [22] | Xu, Z.-H.; Wang, Y.; Wang, Y.; Li, J.-Y.; Luo, W.-F.; Wu, W.-N.; Fan, Y.-C. Spectrochim. Acta, Part A 2019, 212, 146. |
| [23] | Wang, Z.; Yang, J.; Yang, Y.; Xu, X.; Li, M.; Zhang, Y.; Fang, H.; Xu, H.; Wang, S. Chin. J. Org. Chem. 2018, 38, 1401 (in Chinese). |
| ( 王忠龙, 杨金来, 杨益琴, 徐徐, 李明新, 张燕, 方华, 徐海军, 王石发, 有机化学 , 2018, 38, 1401.) | |
| [24] |
Shekari, Z.; Younesi, H.; Heydari, A.; Tajbakhsh, M.; Chaichi, M.; Shahbazi, J. A.; Saberi, D. Chemosensors 2017, 5, 26.
doi: 10.3390/chemosensors5030026 |
| [25] | Shi, B. B.; Zhang, P.; Wei, T.-B.; Yao, H.; Qi, L.; Liu, J.; Zhang, Y.-M. Tetrahedron 2013, 16. 7981. |
| [26] | Wang, L.-Y.; Fang, G.-P.; Cao, D.-R. Sens. Actuators, B 2015, 207, 849. |
| [27] |
Abrego, Z.; Unceta, N.; Sanchez, A.; Gomez-Caballero, A.; Berrio-Ochoa, L. M.; Goicolea, M. A.; Barrio, J. Analyst 2017, 142, 1157.
doi: 10.1039/C7AN00049A |
| [28] | Asadpour-Zeynali, K.; Amini, R. Sens. Actuators, B 2017, 246, 961. |
| [29] | Kallithrakas-Kontos, N.; Foteinis, S.C. Anal. Chem. 2016, 12, 22. |
| [30] | Song, C. Y.; Yang, B. Y.; Yang, Y. J.; Wang, L.H. Sci. China: Chem. 2016, 59, 16. |
| [31] | Sun, Z. L.; Du, J. J; Jing, C.Y. . J. Environ. Sci. 2016, 39, 134. |
| [32] | West, M.; Ellis, A. T.; Potts, P. J.; Streli, C.; Vanhoof, C.; Wobrauschek, P. J. Anal. At. Spectrom. 2016, 31, 1706. |
| [33] | van den, Berg. C. M. G. , Anal. Chem. 2006, 78, 156. |
| [34] |
Lunvongsa, S.; Oshima, M.; Motomizu, S. Talanta 2006, 68, 969.
doi: 10.1016/j.talanta.2005.06.067 |
| [35] | Graser, C. H.; Banik, N. I.; Bender, K. A.; Lagos, M.; Marquardt, C. M.; Marsac, R.; Montoya, V.; Geckeis, H. Anal. Chem. 2015, 87, 9786. |
| [36] | Liu, R.; Zeng, J. Chin. J. Org. Chem. 2017, 37, 3274 (in Chinese). |
| ( 刘瑞姣, 曾竟, 有机化学 , 2017, 37, 3274.) | |
| [37] | Zhang, M.; Xiao, H.; Han, Z.; Yang, L.; Wu, X. Chin. J. Org. Chem. 2018, 38, 926 (in Chinese). |
| ( 张敏, 肖慧丰, 韩志湘, 仰榴青, 吴向阳, 有机化学, 2018, 38, 926.) | |
| [38] |
Zhang, H.; Nie, C.; Wang, J.; Guan, R.; Cao, D. Talanta 2019, 195, 713.
doi: 10.1016/j.talanta.2018.11.082 |
| [39] |
Behera, K. C.; Bag, B. Dyes Pigm. 2016, 135, 143.
doi: 10.1016/j.dyepig.2016.04.034 |
| [40] | Zhang, X.; Man Lai, E. S.; Martin-Aranda, R.; Yeung, K.L. Appl. Catal. A 2004, 261, 109. |
| [41] | Chen, Z.; Cai, D.; Mou, D.; Yan, Q.; Sun, Y.; Pan, W.; Wan, Y.; Song, H.; Yi, W. Bioorg. Med. Chem. 2014, 22, 3279. |
| [42] | Wang, J.; Zhang, L.; Qi, Q.; Li, S.; Jiang, Y. Anal. Methods 2013, 5, 608. |
| [43] | Zhang, Y.-M.; Zhu, W.; Qu, W.-J.; Zhong, K.-P.; Chen, X.-P.; Yao, H.; Wei, T.-B.; Lin, Q. Chem. Commun. 2018, 54, 4549. |
| [44] | Qi, L.; Fan, Y.-Q.; Gong, G.-F.; Mao, P.-P.; Wang, J.; Guan, X.-W.; Liu, J.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. ACS Sustainable Chem. Eng. 2018, 6, 8775. |
| [45] | Li, X.; Lin, Q.; Qu, W-J.; Li, Q.; Chen, X.-B.; Li, W.-T.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. Chin. J. Org. Chem. 2017, 37, 889 (in Chinese). |
| ( 李翔, 林奇, 曲文娟, 李乔, 程晓斌, 李文婷, 张有明, 姚虹, 魏太保, 有机化学, 2017, 37, 889.) | |
| [46] | Li, W.-T.; Qu, W-J.; Zhang, H.-L.; Li, X.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. Chin. J. Org. Chem. 2017, 37, 2619 (in Chinese). |
| ( 李文婷, 曲文娟, 张海丽, 李翔, 林奇, 姚虹, 张有明, 魏太保, 有机化学, 2017, 37, 2619.) | |
| [47] |
Ma, X.-Q.; Wang, Y.; Wei, T.-B.; Qi, L.-H.; Jiang, X.-M.; Ding, J.-D.; Zhu, W.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. Dyes Pigm. 2019, 164, 279.
doi: 10.1016/j.dyepig.2019.01.049 |
| [48] | Kim, H. N.; Ren, W. X.; Kim, J. S.; Yoon, J. Chem. Soc. Rev. 2012, 41, 3210. |
| [49] | Shi, W.; Zhao, S.; Su, Y.; Hui, Y.; Xie, Z.F. New J. Chem. 2016, 40, 7814. |
| [50] | Lin, L.; Lv, J.; Ji, Y.; Feng, J.; Liu, Y.; Wang, Z.; Zhang, W. Anal. Lett. 2013, 46, 2890. |
| [51] | Wang, L.; Zeng, R.; Li, C.; Qiao, R. Colloids Surf., B 2009, 74, 284. |
| [1] | 李焕清, 陈兆华, 陈祖佳, 邱琪雯, 张又才, 陈思鸿, 汪朝阳. 基于有机小分子的汞离子荧光探针研究进展[J]. 有机化学, 2023, 43(9): 3067-3077. |
| [2] | 李阳阳, 孙小飞, 胡晓玲, 任源远, 钟克利, 燕小梅, 汤立军. 三苯胺衍生物的合成及其基于聚集诱导发光(AIE)机理对汞离子“OFF-ON”荧光识别[J]. 有机化学, 2023, 43(1): 320-325. |
| [3] | 鞠立鑫, 邵琦, 陆临川, 陆鸿飞. 基于嘌呤席夫碱荧光探针检测Al3+及细胞实验应用[J]. 有机化学, 2022, 42(6): 1706-1712. |
| [4] | 薛松松, 解正峰, 褚义成, 岳永双, 石伟, 周家斌. 高选择性快速检测Hg2+的磺酰腙型探针的合成及其在吸附和HeLa细胞中的应用[J]. 有机化学, 2021, 41(3): 1138-1145. |
| [5] | 马素芳, 余强, 陆利, 李丽红, 刘文, 武志芳, 李思进. 亚铁离子荧光探针的研究进展[J]. 有机化学, 2021, 41(1): 229-240. |
| [6] | 马学林, 韩利民, 张骁勇, 郝占忠, 杨威, 张玉恒, 王丽. 多响应锆基金属有机框架荧光传感器对Fe3+,Cr2O72-离子和有机小分子的识别[J]. 有机化学, 2020, 40(9): 2938-2948. |
| [7] | 张继东, 詹妍, 李胡月雯, 齐怡, 王瑞鹏, 孟莉. 硒化合物荧光传感器研究进展[J]. 有机化学, 2020, 40(7): 1847-1859. |
| [8] | 马学林, 韩利民, 张骁勇, 张玉恒, 王丽, 杨坤, 冀婕. 三嗪衍生物荧光探针对Zr4+,Fe3+和丙酮的识别[J]. 有机化学, 2020, 40(6): 1745-1751. |
| [9] | 曹西颖, 罗时荷, 杨崇岭, 肖颖, 李晓燕, 张钧如, 汪朝阳. 有机聚合物荧光传感器的研究进展[J]. 有机化学, 2020, 40(12): 4046-4059. |
| [10] | 常永新, 李白, 郭淼, 蔡永红, 徐括喜. 一种新型连续检测镉离子和焦磷酸阴离子的逻辑门荧光传感器及其细胞成像研究[J]. 有机化学, 2019, 39(9): 2485-2491. |
| [11] | 张继东, 张俊, 严瞻, 谢娟平. 基于有机小分子的三磷酸腺苷荧光传感器研究进展[J]. 有机化学, 2019, 39(11): 3051-3064. |
| [12] | 刘钰, 汤永星, 罗月阳, 朱叶婷, 杨进, 魏欣雨, 刘雄, 赵云辉. 含巴比妥结构荧光探针的合成及其应用研究[J]. 有机化学, 2019, 39(10): 2980-2984. |
| [13] | 付怡, 唐辉, 刘泽, 张万轩, 任君. 一种氟离子荧光传感器分子的合成及性能研究[J]. 有机化学, 2018, 38(7): 1806-1810. |
| [14] | 李翔, 林奇, 曲文娟, 李乔, 程晓斌, 李文婷, 张有明, 姚虹, 魏太保. 一种咪唑并吩嗪内酰胺反应型识别氰离子的荧光探针[J]. 有机化学, 2017, 37(4): 889-895. |
| [15] | 肖立伟, 任萍, 景学敏, 任丽磊, 李政, 戴富才. 1,2,3-三唑类化合物在分子识别中的应用[J]. 有机化学, 2017, 37(12): 3085-3095. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||