有机化学 ›› 2019, Vol. 39 ›› Issue (10): 2821-2828.DOI: 10.6023/cjoc201904058 上一篇 下一篇
研究论文
收稿日期:2019-04-24
修回日期:2019-05-26
发布日期:2019-06-12
通讯作者:
刘运奎
E-mail:ykuiliu@zjut.edu.cn
基金资助:
Zheng Limeng, Shi Dongdong, Bao Hanyang, Liu Yunkui*( )
)
Received:2019-04-24
Revised:2019-05-26
Published:2019-06-12
Contact:
Liu Yunkui
E-mail:ykuiliu@zjut.edu.cn
Supported by:文章分享
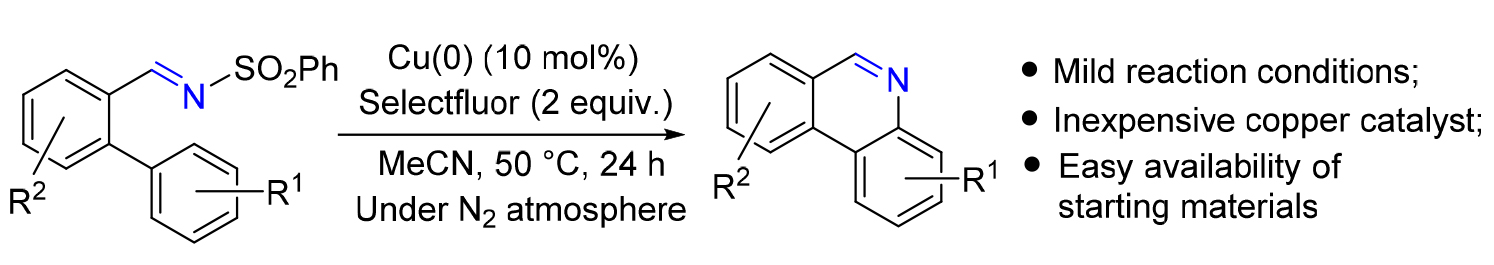
发展了Cu(0)/Selectfluor体系催化的邻芳基磺酰亚胺的串联环化/芳构化反应, 于温和的反应条件下以中等到良好的产率简便、高效地构建了一系列6H-菲啶类化合物. 机理研究表明, 反应的关键步骤经历了由Cu(0)/Selectfluor体系现场原位产生XCuOH (X=F, BF4)物种, 进而诱导对C=N键的羟铜化反应和分子内C—H键胺化反应, 从而合成了6H-菲啶类化合物.
郑立孟, 施冬冬, 鲍汉扬, 刘运奎. Cu(0)/Selectfluor体系催化的邻芳基磺酰亚胺的串联环化/芳构化反应:一种简便合成6H-菲啶的方法[J]. 有机化学, 2019, 39(10): 2821-2828.
Zheng Limeng, Shi Dongdong, Bao Hanyang, Liu Yunkui. Copper(0)/Selectfluor System-Catalyzed Tandem Annulation/Aromatization of o-Aryl Benzenesulfonylimides:A Facile Synthesis of 6H-Phenanthridines[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2821-2828.
| Entry | Catalyst | Oxidant | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | Cu(0) | Selectfluor | MeCNc | 50 | 63 |
| 2 | Cu(0) | Selectfluor | Anhydrous MeCN | 50 | 15 |
| 3 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 84 (80)d |
| 4 | Cu(0) | Selectfluor | MeCN/H2O (V:V=800:1) | 50 | 82 |
| 5 | Cu(0) | Selectfluor | MeCN/H2O (V:V=250:1) | 50 | 72 |
| 6 | Cu(0) | Selectfluor | Solvente/H2O (V:V=500:1) | 50 | 0 |
| 7 | CuI | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 55 |
| 8 | CuCl | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 63 |
| 9 | CuBr | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 77 |
| 10 | Cu2O | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 72 |
| 11 | CuCl2 | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 47 |
| 12 | Cu(OAc)2 | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 48 |
| 13 | — | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 14 | Cu(0) | — | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 15 | Cu(0) | NFSIf | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 16 | Cu(0) | Other oxidantg | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 17 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 25 | 69 |
| 18 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 80 | 80 |
| 19 | Cu(0)h | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 76 |
| 20 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 46i, 82j |
| Entry | Catalyst | Oxidant | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | Cu(0) | Selectfluor | MeCNc | 50 | 63 |
| 2 | Cu(0) | Selectfluor | Anhydrous MeCN | 50 | 15 |
| 3 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 84 (80)d |
| 4 | Cu(0) | Selectfluor | MeCN/H2O (V:V=800:1) | 50 | 82 |
| 5 | Cu(0) | Selectfluor | MeCN/H2O (V:V=250:1) | 50 | 72 |
| 6 | Cu(0) | Selectfluor | Solvente/H2O (V:V=500:1) | 50 | 0 |
| 7 | CuI | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 55 |
| 8 | CuCl | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 63 |
| 9 | CuBr | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 77 |
| 10 | Cu2O | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 72 |
| 11 | CuCl2 | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 47 |
| 12 | Cu(OAc)2 | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 48 |
| 13 | — | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 14 | Cu(0) | — | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 15 | Cu(0) | NFSIf | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 16 | Cu(0) | Other oxidantg | MeCN/H2O (V:V=500:1) | 50 | 0 |
| 17 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 25 | 69 |
| 18 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 80 | 80 |
| 19 | Cu(0)h | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 76 |
| 20 | Cu(0) | Selectfluor | MeCN/H2O (V:V=500:1) | 50 | 46i, 82j |
| [1] | (a) Cushman, M.; Mohan, P.; Smith, E.C. R. J. Med. Chem. 1984, 27, 544. |
| (b) Fang, S. D.; Wang, L. K.; Hecht, S.M. J. Org. Chem. 1993, 5025, 58. | |
| (c) Lynch, M. A.; Duval, O.; Sukhanova, A.; Devy, J.; Mackay, S. P.; Waigh, R. D.; Nabiev, I. Bioorg. Med. Chem. Lett. 2001, 11, 2643. | |
| (d) Bernardo, P. H.; Wan, K. F.; Sivaraman, T.; Xu, J.; Moore, F. K.; Hung, A. W.; Mok, H. Y. K.; Yu, V. C.; Chai, C. L. L. J. Med. Chem. 2008, 51, 6699. | |
| (e) Zhu, S.; Ruchelman, A. L.; Zhou, N.; Liu, A.; Liu, L. F.; LaVoie, E.J. Bioorg. Med. Chem. 2005, 1, 6782. | |
| (f) Li, D.; Zhao, B.; Sim, S.-P.; Li, T.-K.; Liu, A.; Liu, L. F.; Edmond, J.; LaVoie, E. J. Bioorg. Med. Chem. 2003, 11, 521. | |
| (g) Tsukamoto, H.; Kondo, S.; Mukudai, Y.; Nagumo, T.; Yasuda, A.; Kurihara, Y.; Kamatani, T.; Shintani, S. Anticancer Res. 2011, 31, 2841. | |
| [2] | (a) Cushman, M.; Mohan, P; Smith, E.C. R. . J. Med. Chem. 1984, 27, 544. |
| (b) Fang, S. D.; Wang, L. K.; Hecht, S.M. J. Org. Chem. 1993, 58, 5025. | |
| (c) Lynch, M. A.; Duval, O.; Sukhanova, A.; Devy, J.; Mackay, S. P.; Waigh, R. D.; Nabiev, I. Bioorg. Med. Chem. Lett. 2001, 11, 2643. | |
| (d) Bernardo, P. H.; Wan, K. F.; Sivaraman, T.; Xu, J.; Moore, F. K.; Hung, A. W.; Mok, H. Y. K.; Yu, V. C.; Chai, C. L. L. J. Med. Chem.2008, 51, 6699. | |
| (e) Zhu, S.; Ruchelman, A. L.; Zhou, N.; Liu, A.; Liu, L. F.; LaVoie, E.J. Bioorg. Med. Chem. 2005, 13, 6782. | |
| (f) Li, D.; Zhao, B.; Sim, S.-P.; Li, T.-K.; Liu, A.; Liu, L. F.; Edmond, J.; LaVoie, E.J. Bioorg. Med. Chem. 2003, 11, 521. | |
| (g) Tsukamoto, H.; Kondo, S.; Mukudai, Y.; Nagumo, T.; Yasuda, A.; Kurihara, Y.; Kamatani, T.; Shintani, S. Anticancer Res. 2011, 31, 2841. | |
| [3] | (a) Stevens, N.; O’Connor, N.; Vishwasrao, H.; Samaroo, D.; Kandel, E. R.; Akins, D. L.; Drain, C. M.; Rurro, N.J. J. Am. Chem. Soc. 2008, 130, 7182. |
| (b) Bondarev, S. L.; Knyukshto, V. N.; Tikhomirov, S. A.; Pyrko, A.N. Opt. Spectrosc. 2006, 100, 386. | |
| (c) Zhang, J.; Lakowicz, J.R. J. Phys. Chem. B. 2005, 109, 8701. | |
| [4] | For selected recent reviews, see: (a) Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505. |
| (b) Hayashi, H.; Kaga, A.; Chiba, S. J. Org. Chem. 2017, 82, 11981. | |
| (c) Hu, B.; DiMagno, S.G. Org. Biomol. Chem. 2015, 13, 3844. | |
| [5] |
(a) Pictet, A.; Hubert, A. Ber. Dtsch. Chem. Ges. 1896, 29, 1182.
doi: 10.1002/(ISSN)1099-0682 |
|
(b) Morgan, T.; Walls, L. P. J. Chem. Soc., 1931, 2447.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(c) Chinnagolla, R. K.; Jeganmohan, M. Chem. Commun. 2014, 50. 2442.
doi: 10.1002/(ISSN)1099-0682 |
|
| [6] |
(a) Ge, J.; Wang, X.; Liu, T.; Shi, Z.; Xiao, Q.; Yin, D. RSC Adv. 2016, 6, 19571.
doi: 10.1039/C6RA00249H |
|
(b) Sahoo, M. K.; Midya, S. P.; Landge, V. G.; Balaraman, E. Green Chem. 2017, 19, 2111.
doi: 10.1039/C6RA00249H |
|
|
(c) Candito, D. A.; Lautens, M. Angew. Chem. Int. Ed. 2009, 48, 6713.
doi: 10.1039/C6RA00249H |
|
|
(d) Xu, Z.; Hang, Z.; Liu, Z.-Q. Org. Lett. 2016, 18, 4470.
doi: 10.1039/C6RA00249H |
|
|
(e) Li, Z.; Fan, F.; Yang, J.; Liu, Z.-Q. Org. Lett. 2014, 16, 3396.
doi: 10.1039/C6RA00249H |
|
|
(f) Xu, Z.; Yan, C.; Liu, Z.-Q. Org. Lett. 2014, 16, 5670.
doi: 10.1039/C6RA00249H |
|
| [7] | (a) Dai, Q.; Yun, J.-T.; Feng, X.; Jiang, Y.; Yang, H.; Cheng, J. Adv. Synth. Catal. 2014, 356, 3341. |
| (b) Sha, W.; Yu, J.-T.; Jiang, Y.; Yang, H.; Cheng, J. Chem. Commun. 2014, 50, 9179. | |
| (c) Tu, H.-Y.; Liu, Y.-R.; Chu, J.-J.; Hu, B.-L.; Zhang, X.-G. J. Org. Chem. 2014, 79, 9907. | |
| (d) Jiang, H.; An, X.; Tong, K.; Zhang, Y.; Yu, S. Angew. Chem. Int. Ed. 2015, 54, 4055. | |
| (e) Cao, J.-J.; Zhu, T.-H.; Wang, S.-Y.; Gu, Z.-Y.; Wang, X.; Ji, S.-J. Chem. Commun. 2014, 50, 6439. | |
| (f) Zhang, B.; Mück-Lichtenfeld, C.; Daniliuc, C. G.; Studer, A. Angew. Chem. Int. Ed. 2013, 52, 10792. | |
| (g) Sun, X.; Yu, S. Chem. Commun. 2016, 52, 10898. | |
| (h) Wang, Y.-F.; Lonca, G. H.; Runigo, M. L.; Chiba, S. Org. Lett., 2014, 16, 4272. | |
| (i) Lu, L.; Zhou, B.; Jin, H.; Liu, Y. Chin. J. Org. Chem. 2019, 39, 515.(in Chinese). | |
| ( 陆露露, 周丙伟, 金红卫, 刘运奎, 有机化学, 2019, 39, 515.) | |
| (j) Shi, D.; Bao, H.; Xu, Z.; Liu, Y. Chin. J. Org. Chem. 2017, 37, 1290(in Chinese). | |
| ( 施冬冬, 鲍汉扬, 徐峥, 刘运奎, 有机化学, 2017, 37, 1290.) | |
| [8] | (a) Evoniuk, C. J.; dos Passos, Gomes, Hill, G.; S, P.; Fujita, S.; Hanson, K.; Alabugin, I.V. J. Am. Chem. Soc. 2017, 139, 16210. |
| (b) Evoniuk, C. J.; Hill, S. P.; Hanson, K.; Alabugin, I.V. Chem. Commun. 2016, 52, 7138. | |
| (c) Zhang, L.; Ang, G. Y.; Chiba, S. Org. Lett. 2010, 12, 3682. | |
| [9] | (a) Liu, Y.-Y.; Song, R.-J.; Wu, C.-Y.; Gong, L.-B.; Hu, M.; Wang, Z.-Q.; Xie, Y.-X.; Li, J.-H. Adv. Synth. Catal. 2012 354, 347. |
| (b) Borah, A.; Gogoi, P. Eur. J. Org. Chem. 2016, 2200. | |
| (c) Han, W.; Zhou, X.; Yang, S.; Xiang, G.; Cui, B.; Chen, Y. J. Org. Chem. 2015 80, 11580. | |
| (d) Maestri, G.; Larraufie, M.-H.; Derat, É.; Ollivier, C.; Fensterbank, L.; Lacȏte, E.; Malacria, M. Org. Lett. 2010 12, 5692. | |
| (e) Portela-Cubillo, F.; Scott, J. S.; Walton, J.C. J. Org. Chem. 2008 73, 5558. | |
| (f) Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Angew. Chem. Int. Ed. 2015 54, 4055. | |
| (g) An, X.-D.; Yu, S. Org. Lett. 2015 17, 2692. | |
| (h) Ghosh, M.; Ahmed, A.; Singha, R.; Ray, J.K. Tetrahedron Lett. 2015 56, 353. | |
| (i) Portela, C. F.; Scanlan, E. M.; Scott, J. S.; Walton, J.C. Chem. Commun. 2008 4189. | |
| (j) Tummatorn, J.; Krajangsri, S.; Norseeda, K.; Thongsornkleeb, C.; Ruchirawat, S. Org. Biomol. Chem. 2014 12, 5077. | |
| (k) Budén, M.; Dorn, V. B.; Gamba, M.; Píerini, A. B.; Rossi, R.A. J. Org. Chem. 2010 75, 2206. | |
| (l) Linsenmeier, A. M.; Williams, C. M.; Bräse, S. J. Org. Chem. 2011 76, 9127. | |
| (m) McBurney, R. T.; Slawin, A. M. Z.; Smart, L. A.; Yu, Y.; Walton, J.C. Chem. Commun. 2011 47, 7974. | |
| (n) Hofstra, J. L.; Grassbaugh, B. R.; Tran, Q. M.; Armada, N. R.; de Lijser, H.J. P. J. Org. Chem. 2015 80, 256. | |
| (o) Chen, W.-L.; Chen, C.-Y.; Chen, Y.-F.; Hsieh, J.-C. Org. Lett. 2015 17, 1613. | |
| [10] | Selected reviews: |
| (a) Yin, G.; Mu, X.; Liu, G. Acc. Chem. Res. 2016, 49, 2413. | |
| (b) Egami, H.; Sodeoka, M. Angew. Chem. Int. Ed. 2014, 53, 8294. | |
| (c) Shimizu, Y.; Kanai, M. Tetrahedron Lett. 2014, 55, 3727. | |
| (d) McDonald, R.; Liu, G.; Stahl, S.S. Chem. Rev. 2011, 111, 2981. | |
| (e) Zeni, G.; Larock, R.C. Chem. Rev. 2006, 106, 4644. | |
| (f) Vlaar, T.; Ruijter, E.; Orru, R. Adv. Synth. Catal. 2011, 353, 809. | |
| (g) Giri, R.; Shekhar, K.C. J. Org. Chem. 2018, 83, 3013. | |
| (h) Chemler, S. R.; Bovino, M.T. ACS Catal. 2013, 3, 1076. | |
| [11] | Addition of XCuOH to carbon-carbon multiple bonds, see: |
| (a) Zhang, W.; Zhang, J.; Liu, Y.; Xu, Z. Synlett. 2013, 24, 2709. | |
| (b) Zhang, J.; Wu, D.; Chen, X.; Liu, Y.; Xu, Z. J. Org. Chem. 2014, 79, 4799. | |
| (c) Zhang, J.; Wang, H.; Ren, S.; Zhang, W.; Liu, Y. Org. Lett. 2015, 17, 2920. | |
| (d) Zhang, J.; Zhang, H.; Shi, D.; Jin, H.; Liu, Y. Eur. J. Org. Chem. 2016, 5545. | |
| (e) Bao, H.; Xu, Z.; Wu, D.; Zhang, H.; Jin, H.; Liu, Y. J. Org. Chem. 2017, 82, 109. | |
| [12] |
Addition of XCuOH to C=O bonds, see: (f) Zhang, J.; Shi, D.; Zhang, H.; Xu, Z.; Bao, H.; Jin, H.; Liu, Y. Tetrahedron 2017, 73, 154.
doi: 10.1016/j.tet.2016.11.069 |
| [13] | (a) Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2015, 80, 3243. |
| (b) Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892. | |
| (c) Zhou, W.; Liu, Y.; Yang, Y.; Deng, G.-J. Chem. Commun. 2012, 48, 10678. | |
| [14] | (a) Srimani, D.; Leitus, G.; Ben-David, Y.; Milstein, D. Angew. Chem. Int. Ed. 2014, 53, 11092. and references cited therein. |
| (b) Julia, M.; Paris, J.-M. Tetrahedron Lett. 1973, 14, 4833. | |
| (c) Blakemore, P.R. J. Chem. Soc. Perkin Trans. 2002, 2563. | |
| (d) Chatterjee, B.; Bera, S.; Mondal, D. Tetrahedron: Asymmetry. 2014, 25, 1. | |
| [15] | Yamanaka, M.; Nishida, A.; Nakagawa, M. J. Org. Chem. 2003, 68, 3112. |
| [16] |
Qiu, J.; Wang, L.; Liu, M.; Shen, Q.; Tang, J. Tetrahedron Lett. 2011, 52, 6489.
doi: 10.1016/j.tetlet.2011.09.115 |
| [17] |
Siddiqui, M. A.; Snieckus, V . Tetrahedron Lett. 1988,29, 5463.
doi: 10.1016/S0040-4039(00)80787-X |
| [18] | Keene, B. R. T. J . Chem. Soc. 1965,3032. |
| [19] | Badger, G. M.; Sasse, W. F. H. J . Chem. Soc. 1957,4. |
| [20] |
Maestri, G.; Larraufie, M.-H.; Derat, E.; Ollivier, C.; Fensterbank, L.; Lacote, E.; Malacria, M. Lett. Org. . 2010, 12, 5692.
doi: 10.1021/ol102509n |
| [21] | Coombs, M. M. J . Chem. Soc. 1958,3454. |
| [22] | Arcus, C. L.; Coombs, M. M.; Evans, J. V. J . Chem. Soc. 1956,1498. |
| [23] | Kessar, S. V.; Grupta, Y. P.; Balakrishnan, P.; Sawal, K. K.; Mohammad, T Dutt, M..; J. Org. Chem. 1988, 53, 1708. |
| [1] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [2] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [3] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [4] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [5] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [6] | 陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119. |
| [7] | 鲍志成, 李慕尧, 王剑波. 铜催化芳基重氮乙酸酯与双[(频哪醇)硼基]甲烷的偶联反应[J]. 有机化学, 2023, 43(5): 1808-1814. |
| [8] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [9] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [10] | 李春生, 连晓琪, 陈莲芬. 铜催化亚砜叶立德与邻苯二胺[4+2]环加成反应[J]. 有机化学, 2023, 43(4): 1492-1498. |
| [11] | 刘洋, 黄翔, 王敏, 廖建. 铜催化环酮亚胺与β,γ-不饱和N-酰基吡唑不对称Mannich-Type反应[J]. 有机化学, 2023, 43(4): 1499-1509. |
| [12] | 刘春阳, 李燕, 张前. 铜催化环状烯烃烯丙位C(sp3)—H磺酰化反应研究[J]. 有机化学, 2023, 43(3): 1091-1101. |
| [13] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [14] | 韩彪, 李维双, 陈舒晗, 张泽浪, 赵雪, 张瑶瑶, 朱磊. 铜催化不饱和化合物硅加成反应的研究进展[J]. 有机化学, 2023, 43(2): 555-572. |
| [15] | 许力, 吕兰兰, 王香善. 铜催化烯醇硅醚与芳基亚磺酸钠合成β-酮砜的研究[J]. 有机化学, 2023, 43(10): 3644-3651. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||