有机化学 ›› 2022, Vol. 42 ›› Issue (8): 2390-2405.DOI: 10.6023/cjoc202202039 上一篇 下一篇
综述与进展
收稿日期:2022-02-28
修回日期:2022-04-13
发布日期:2022-04-29
通讯作者:
李筱芳, 张少伟
基金资助:
Sha Li, Yahan Sun, Yankui Meng, Xiaofang Li( ), Shaowei Zhang(
), Shaowei Zhang( )
)
Received:2022-02-28
Revised:2022-04-13
Published:2022-04-29
Contact:
Xiaofang Li, Shaowei Zhang
Supported by:文章分享
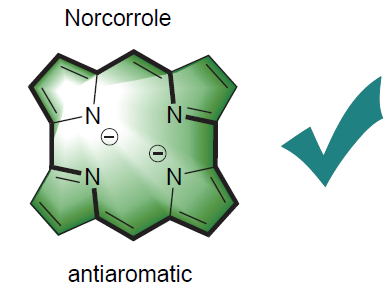
近年来, 去甲咔咯由于显著的顺磁环电流、高单分子电导率、狭窄的HOMO-LUMO能隙、稳定的氧化还原性能以及特有的化学反应性, 引起了科研工作者的广泛关注. 综述了近些年去甲咔咯的不同合成方法, 重点阐述了在插入、取代、氧化还原、延伸π共轭体系四个方面的衍生化反应, 同时对去甲咔咯的发展进行了展望.
李莎, 孙雅涵, 蒙燕葵, 李筱芳, 张少伟. 去甲咔咯的合成及其衍生化反应的进展[J]. 有机化学, 2022, 42(8): 2390-2405.
Sha Li, Yahan Sun, Yankui Meng, Xiaofang Li, Shaowei Zhang. Progress in the Synthesis and Derivatization of Norcorrole[J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2390-2405.


| [1] |
Proft, F. D.; Geerlings, P. Chem. Rev. 2001, 101, 1451.
pmid: 11710228 |
| [2] |
Krygowski, T. M.; Cyrański, M. K. Chem. Rev. 2001, 101, 1385.
pmid: 11710226 |
| [3] |
Schleyer, P. R.; Manoharan, M.; Wang, Z. X.; Kiran, B.; Jiao, H.; Puchta, R.; Hommes, N. E. Org. Lett. 2001, 3, 2465.
pmid: 11483036 |
| [4] |
Liu, J.; Ma, J.; Zhang, K.; Ravat, P.; Machta, P.; Avdoshenko, S.; Hennersdorf, F.; Komber, H.; Pisula, W.; Weigand, J. J.; Popov, A. A.; Berger, R.; Müllen, K.; Feng, X. J. Am. Chem. Soc. 2017, 139, 7513.
doi: 10.1021/jacs.7b01619 |
| [5] |
Breslow, R.; Foss, F. W. J. Phys.: Condens. Matter. 2008, 20, 374104.
|
| [6] |
Nishinaga, T.; Uto, T.; Inoue, R.; Matsuura, A.; Treitel, N.; Rabinovitz, M.; Komatsu, K. Chem.-Eur. J. 2008, 14, 2067.
pmid: 18081128 |
| [7] |
Fan, C.; Mercier, L. G.; Piers, W. E.; Tuononen, H. M.; Parvez, M. J. Am. Chem. Soc. 2010, 132, 9604.
doi: 10.1021/ja105075h |
| [8] |
Breslow, R.; Schneebeli, S. T. Tetrahedron. 2011, 67, 10171.
doi: 10.1016/j.tet.2011.08.008 |
| [9] |
Ghosh, A.; Wasbotten, I. H.; Davis, W.; Swarts, J. C. Eur. J. Inorg. Chem. 2005, 22, 4479.
|
| [10] |
Sessler, J. L.; Tomat, E. Acc. Chem. Res. 2007, 40, 371.
doi: 10.1021/ar600006n |
| [11] |
Aviv-Harel, I.; Gross, Z. Chem.-Eur. J. 2009, 15, 8382.
doi: 10.1002/chem.200900920 pmid: 19630016 |
| [12] |
Nozawa, R.; Yamamoto, K.; Shin, J. Y.; Hiroto, S.; Shinokubo, H. Angew. Chem., Int. Ed. 2015, 54, 8454.
doi: 10.1002/anie.201502666 |
| [13] |
Liu, B.; Yoshida, T.; Li, X.; Stępień, M.; Shinokubo, H.; Chmielewski, P. J. Angew. Chem., Int. Ed. 2016, 55, 13142.
doi: 10.1002/anie.201607237 |
| [14] |
Tu, X. M.; Xie, Q. J.; Jiang, S. Y.; Yao, S. Z. Biosens. Bioelectron. 2007, 22, 2819.
doi: 10.1016/j.bios.2006.11.022 |
| [15] |
Suga, T.; Sugita, S.; Ohshiro, H.; Oyaizu, K.; Nishide. H. Adv. Mater. 2011, 23, 751.
doi: 10.1002/adma.201003525 |
| [16] |
Shin, J. Y.; Yamada, T.; Yoshikawa, H.; Awaga, K.; Shinokubo, H. Angew. Chem., Int. Ed. 2014, 53, 3096.
doi: 10.1002/anie.201310374 |
| [17] |
Deng, K.; Li, X.; Huang, H. Electrochim. Acta 2016, 204, 84.
doi: 10.1016/j.electacta.2016.04.060 |
| [18] |
Fujii, S.; Marqués-González, S.; Shin, J. Y.; Shinokubo, H.; Masuda, T.; Nishino, T.; Arasu, N. P.; Vázquez, H.; Kiguchi, M. Nat. Commun. 2017, 8, 15984.
doi: 10.1038/ncomms15984 |
| [19] |
Ukai, S.; Koo, Y. H.; Fukui, N.; Seki, S.; Shinokubo, H. Dalton Trans. 2020, 49, 14383.
doi: 10.1039/D0DT03143G |
| [20] |
Bröring, M.; Köhler, S.; Kleeberg, C. Angew. Chem., Int. Ed. 2008, 47, 5658.
doi: 10.1002/anie.200801196 |
| [21] |
Ito, T.; Hayashi, Y.; Shimizu, S.; Shin, J. Y.; Kobayashi, N.; Shinokubo, H. Angew. Chem., Int. Ed. 2012, 51, 8542.
doi: 10.1002/anie.201204395 |
| [22] |
Yoshida, T.; Sakamaki, D.; Seki, S.; Shinokubo, H. Chem. Commun. 2017, 53, 1112.
doi: 10.1039/C6CC09444A |
| [23] |
Liu, S.; Tanaka, H.; Nozawa, R.; Fukui, N.; Shinokubo, H. Chem.-Eur. J. 2019, 25, 7618.
doi: 10.1002/chem.201901292 |
| [24] |
Yoshida, T.; Takahashi, K.; Ide, Y.; Kishi, R.; Fujiyoshi, J.; Lee, S.; Hiraoka, Y.; Kim, D.; Nakano, M.; Ikeue, T.; Yamada, H.; Shinokubo, H. Angew. Chem., Int. Ed. 2018, 57, 2209.
doi: 10.1002/anie.201712961 |
| [25] |
Murakami, K.; Yamamoto, Y.; Yorimitsu, H.; Osuka, A. Chem.-Eur. J. 2013, 19, 9123.
doi: 10.1002/chem.201301146 pmid: 23740546 |
| [26] |
Yonezawa, T.; Shafie, S. A.; Hiroto, S.; Shinokubo, H. Angew. Chem., Int. Ed. 2017, 56, 11822.
doi: 10.1002/anie.201706134 |
| [27] |
Zhang, S.; Zhang, D.; Liebeskind, L. S. J. Org. Chem. 1997, 62, 2312.
doi: 10.1021/jo9700078 |
| [28] |
Yoshida, T.; Shafie, S. A.; Kawashima, H.; Fukui, N.; Shinokubo, H. Org. Lett. 2021, 23, 2826.
doi: 10.1021/acs.orglett.1c00823 |
| [29] |
Kido, H.; Shin, J. Y.; Shinokubo, H. Angew. Chem., Int. Ed. 2013, 52, 13727.
doi: 10.1002/anie.201306905 |
| [30] |
Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. J. Am. Chem. Soc. 1999, 121, 9722.
doi: 10.1021/ja9925305 |
| [31] |
Fukuoka, T.; Uchida, K.; Sung, Y. M.; Shin, J. Y.; Ishida, S.; Lim, J. M.; Hiroto, S.; Furukawa, K.; Kim, D.; Iwamoto, T.; Shinokubo, H. Angew. Chem., Int. Ed. 2014, 53, 1506.
doi: 10.1002/anie.201309921 |
| [32] |
Liu, S. Y.; Fukuoka, T.; Fukui, N.; Shin, J. Y.; Shinokubo, H. Org. Lett. 2020, 22, 4400.
doi: 10.1021/acs.orglett.0c01402 |
| [33] |
Ren, D.; Smaga, O.; Fu, X.; Li, X.; Pawlicki, M.; Koniarz, S.; Chmielewski, P. J. Org. Lett. 2021, 23, 1032.
doi: 10.1021/acs.orglett.0c04227 |
| [34] |
Horie, M.; Hayashi, Y.; Yamaguchi, S.; Shinokubo, H. Chem.-Eur. J. 2012, 18, 5919.
doi: 10.1002/chem.201200485 pmid: 22454294 |
| [35] |
Li, X.; Sun, Y. H.; Yu, X. Y.; Tan, J. X. CN 112174972, 2021.
|
| [36] |
Yoshida, T.; Shinokubo, H. Mater. Chem. Front. 2017, 1, 1853.
doi: 10.1039/C7QM00176B |
| [37] |
Li, X.; Liu, B.; Yi, P.; Yi, R.; Yu, X.; Chmielewski, P. J. J. Org. Chem. 2011, 76, 2345.
doi: 10.1021/jo200040x |
| [38] |
Liu, B.; Li, X.; Zhang, J.; Chmielewski, P. J. Org. Biomol. Chem. 2013, 11, 4831.
doi: 10.1039/c3ob40754c |
| [39] |
Ren, D.; Fu, X.; Li, X.; Koniarz, S.; Chmielewski, P. J. Org. Chem. Front. 2019, 6, 2924.
doi: 10.1039/C9QO00679F |
| [40] |
Koley, D.; Colón, O. C.; Savinov, S. N. Org. Lett. 2009, 11, 4172.
doi: 10.1021/ol901731w |
| [41] |
Deng, Z.; Li, X.; Stępień, M.; Chmielewski, P. J. Chem.-Eur. J. 2016, 22, 4231.
doi: 10.1002/chem.201504584 |
| [42] |
Kawashima, H.; Hiroto, S.; Shinokubo, H. J. Org. Chem. 2017, 82, 10425.
doi: 10.1021/acs.joc.7b01899 pmid: 28901152 |
| [43] |
Li, S.; Smaga, O.; Sun, Y.; Li, X.; Pawlicki, M.; Sukniewiczb, M.; Chmielewski, P. J. Org. Chem. Front. 2021, 8, 3639.
doi: 10.1039/D1QO00621E |
| [44] |
Shafie, S. A.; Kawashima, H.; Miyake, Y.; Shinokubo, H. ChemPlusChem 2019, 84, 623.
doi: 10.1002/cplu.201900068 pmid: 31944005 |
| [45] |
Liu, B.; Li, X.; Stępień, M.; Chmielewski, P. J. Chem.-Eur. J. 2015, 21, 7790.
doi: 10.1002/chem.201500736 |
| [46] |
Nozawa, R.; Yamamoto, K.; Hisaki, I.; Shin, J. Y.; Shinokubo, H. Chem. Commun. 2016, 52, 7106.
doi: 10.1039/C6CC02918C |
| [47] |
Whitlock, H. W.; Hanauer, R.; Oester, M. Y.; Bower, B. K. J. Am. Chem. Soc. 1969, 91, 7485.
doi: 10.1021/ja01054a044 |
| [48] |
Ukai, S.; Fukui, N.; Ikeue, T.; Shinokubo, H. Chem. Lett. 2022, 51, 590.
doi: 10.1246/cl.220122 |
| [49] |
Silva, A. M. G.; Tomé, A. C.; Neves, M. G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S. J. Org. Chem. 2005, 70, 2306.
pmid: 15760219 |
| [50] |
Li, X.; Zhuang, J.; Li, Y.; Liu, H.; Wang, S.; Zhu, D. Tetrahedron Lett. 2005, 46, 1555.
doi: 10.1016/j.tetlet.2004.12.138 |
| [51] |
Li, X.; Chmielewski, P. J.; Xiang, J.; Xu, J.; Jiang, L.; Li, Y.; Liu, H.; Zhu, D. J. Org. Chem. 2006, 71, 9739.
doi: 10.1021/jo0618268 |
| [52] |
Fu, X.; Meng, Y.; Li, X.; Stępień, M.; Chmielewski, P. J. Chem. Commun. 2018, 54, 2510.
doi: 10.1039/C8CC00447A |
| [53] |
Yokoi, H.; Wachi, N.; Hiroto, S.; Shinokubo, H. Chem. Commun. 2014, 50, 2715.
doi: 10.1039/C3CC48738E |
| [54] |
Li, X.; Meng, Y.; Yi, P.; Stępień, M.; Chmielewski, P. J. Angew. Chem., Int. Ed. 2017, 56, 10810.
doi: 10.1002/anie.201705715 |
| [55] |
Tanaka, T.; Osuka, A. Chem. Soc. Rev. 2015, 44, 943.
doi: 10.1039/C3CS60443H |
| [56] |
Tanaka, T.; Osuka, A. Chem.-Eur. J. 2018, 24, 17188.
doi: 10.1002/chem.201802810 |
| [57] |
Liu, S. Y.; Kawashima, H.; Fukui, N.; Shinokubo, H. Chem. Commun. 2020, 56, 6846.
doi: 10.1039/D0CC02543G |
| [58] |
Nozawa, R.; Tanaka, H.; Cha, W. Y.; Hong, Y.; Hisaki, I.; Shimizu, S.; Shin, J. Y.; Kowalczyk, T.; Irle, S.; Kim, D.; Shinokubo, H. Nat. Commun. 2016, 7, 13620.
doi: 10.1038/ncomms13620 pmid: 27901014 |
| [59] |
Nozawa, R.; Kim, J.; Oh, J.; Lamping, A.; Wang, Y.; Shimizu, S.; Hisaki, I.; Kowalczyk, T.; Fliegl, H.; Kim, D.; Shinokubo, H. Nat. Commun. 2019, 10, 3576.
doi: 10.1038/s41467-019-11467-4 pmid: 31395873 |
| [60] |
Kawashima, H.; Ukai, S.; Nozawa, R.; Fukui, N.; Fitzsimmons, G.; Kowalczyk, T.; Fliegl, H.; Shinokubo, H. J. Am. Chem. Soc. 2021, 143, 10676.
doi: 10.1021/jacs.1c04348 |
| [61] |
Ukai, S.; Takamatsu, A.; Nobuoka, M.; Tsutsui, Y.; Fukui, N.; Ogi, S.; Seki, S.; Yamaguchi, S.; Shinokubo, H. Angew. Chem., Int. Ed. 2022, 61, e202114230.
|
| [1] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [5] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [6] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [7] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [8] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [9] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [10] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [11] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [12] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [13] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [14] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| [15] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||