Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (5): 2063-2073.DOI: 10.6023/cjoc202010042 Previous Articles Next Articles
ARTICLES
王超超, 刘会, 赵微, 李攀, 冀庐莎, 柳仁民, 雷康*( ), 徐效华
), 徐效华
收稿日期:2020-10-30
修回日期:2020-11-25
发布日期:2021-02-22
通讯作者:
雷康
作者简介:基金资助:
Chaochao Wang, Hui Liu, Wei Zhao, Pan Li, Lusha Ji, Renmin Liu, Kang Lei*( ), Xiaohua Xu
), Xiaohua Xu
Received:2020-10-30
Revised:2020-11-25
Published:2021-02-22
Contact:
Kang Lei
About author:Supported by:Share
Chaochao Wang, Hui Liu, Wei Zhao, Pan Li, Lusha Ji, Renmin Liu, Kang Lei, Xiaohua Xu. Synthesis and Herbicidal Activity of 5-(1-Amino-2-phenoxyethylidene)barbituric Acid Derivatives[J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2063-2073.


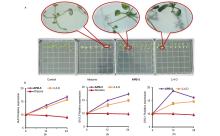

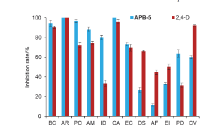

| Compd. | Rate/(g?ha–1) | B. campestris | A. retroflexus | E. crusgalli | D. sanguinalis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
| APB-5 | 750 | 100 | 100 | 100 | 100 | 93.2±2.0 | 33.3±2.4 | 55.1±1.3 | 20.2±1.7 | |||
| 375 | 94.4±3.0 | 100 | 100 | 100 | 73.2±1.7 | 5.1±0.8 | 26.8±2.7 | 4.6±1.1 | ||||
| 187.5 | 77.1±2.3 | 100 | 93.1±2.9 | 100 | 49.6±2.5 | 0 | 17.0±1.8 | 0 | ||||
| 2,4-D | 750 | 100 | 100 | 100 | 100 | 100 | 43.8±2.9 | 92.4±2.1 | 50.0±1.9 | |||
| 375 | 90.5±1.2 | 100 | 100 | 100 | 69.7±3.3 | 20.4±2.0 | 66.1±1.0 | 9.9±2.1 | ||||
| 187.5 | 41.4±2.6 | 100 | 92.4±1.8 | 100 | 55.5±1.1 | 9.9±3.7 | 17.3±2.6 | 0 | ||||
| Compd. | Rate/(g?ha–1) | B. campestris | A. retroflexus | E. crusgalli | D. sanguinalis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
| APB-5 | 750 | 100 | 100 | 100 | 100 | 93.2±2.0 | 33.3±2.4 | 55.1±1.3 | 20.2±1.7 | |||
| 375 | 94.4±3.0 | 100 | 100 | 100 | 73.2±1.7 | 5.1±0.8 | 26.8±2.7 | 4.6±1.1 | ||||
| 187.5 | 77.1±2.3 | 100 | 93.1±2.9 | 100 | 49.6±2.5 | 0 | 17.0±1.8 | 0 | ||||
| 2,4-D | 750 | 100 | 100 | 100 | 100 | 100 | 43.8±2.9 | 92.4±2.1 | 50.0±1.9 | |||
| 375 | 90.5±1.2 | 100 | 100 | 100 | 69.7±3.3 | 20.4±2.0 | 66.1±1.0 | 9.9±2.1 | ||||
| 187.5 | 41.4±2.6 | 100 | 92.4±1.8 | 100 | 55.5±1.1 | 9.9±3.7 | 17.3±2.6 | 0 | ||||
| [1] |
Gross, E. M.; Wolk, C. P.; Jüttner, F. J. Phycol. 1991, 27, 686.
doi: 10.1111/j.0022-3646.1991.00686.x |
| [2] |
Ding, L.; Dahse, H.-M.; Hertweck, C. J. Nat. Prod. 2012, 75, 617.
doi: 10.1021/np2008544 |
| [3] |
Avdović, E. H.; Stojković, D. L.; Jevtić, V. V.; Milenković, D.; Marković, Z. S.; Vuković, N.; Potočňák, I.; Radojević, I. D.; Čomić, L. R.; Trifunović, S. R. Inorg. Chim. Acta 2019, 484, 52.
doi: 10.1016/j.ica.2018.09.014 |
| [4] |
Mladenović, M.; Vuković, N.; Nićiforović, N.; Sukdolak, S.; Solujić, S. Molecules 2009, 14, 1495.
doi: 10.3390/molecules14041495 pmid: 19384281 |
| [5] |
Baldwin, A. G.; Bevan, J.; Brough, D.; Ledder, R.; Freeman, S. Med. Chem. Res. 2018, 27, 884.
doi: 10.1007/s00044-017-2110-8 pmid: 29527108 |
| [6] |
Garmaise, D. L.; Chu, D. T. W.; Bernstein, E.; Inaba, M. J. Med. Chem. 1979, 22, 559.
pmid: 379332 |
| [7] |
Canela, M. D.; Pérez-Pérez, M. J.; Noppen, S.; Sáez-Calvo, G.; Díaz, J. F.; Camarasa, M. J.; Liekens, S.; Priego, E. M. J. Med. Chem. 2014, 57, 3924.
doi: 10.1021/jm401939g |
| [8] |
Ilić, D. R.; Jevtić, V. V.; Radić, G. P.; Arsikin, K.; Ristić, B.; Harhaji-Trajković, L.; Vuković, N.; Sukdolak, S.; Klisurić, O.; Trajković, V.; Trifunović, S. R. Eur. J. Med. Chem. 2014, 74, 502.
doi: 10.1016/j.ejmech.2013.12.051 |
| [9] |
Budzisz, E.; Keppler, B. K.; Giester, G.; Wozniczka, M.; Kufelnicki, A.; Nawrot, B. Eur. J. Inorg. Chem. 2004, 2004, 4412.
doi: 10.1002/(ISSN)1099-0682 |
| [10] |
Budzisz, E.; Małecka, M.; Lorenz, I. P.; Mayer, P.; Kwiecien, R. A.; Paneth, P.; Krajewska, U.; Rozalski, M. Inorg. Chem. 2006, 45, 9688.
doi: 10.1021/ic0605569 |
| [11] |
Budzisz, E.; Brzezinska, E.; Krajewska, U.; Rozalski, M. Eur. J. Med. Chem. 2003, 38, 597.
doi: 10.1016/S0223-5234(03)00086-2 |
| [12] |
Dias, L. C.; Demuner, A. J.; Valente, V. V. M.; Barbosa, L. C. A.; Martins, F. T.; Doriguetto, A. C.; Ellena, J. J. Agric. Food Chem. 2009, 57, 1399.
doi: 10.1021/jf802805f |
| [13] |
Serban, A.; Watson, K. G.; Bird, G. J.; Farquharson, G. J.; Cross, L. E. US 4952722, 1990.
|
| [14] |
Liu, Y. X.; Zhao, H. P.; Wang, Z. W.; Li, Y. H.; Song, H, B.; Riches, H.; Beattie, D.; Gu, Y. C.; Wang, Q. M. Mol. Diversity 2013, 17, 701.
doi: 10.1007/s11030-013-9466-6 |
| [15] |
Batran, R. Z.; Khedr, M. A.; Abdel Latif, N. A.; Abd El Aty, A. A.; Shehata, A. N. J. Mol. Struct. 2019, 1180, 260.
doi: 10.1016/j.molstruc.2018.11.099 |
| [16] |
Lv, P.; Chen, Y. L.; Zhao, Z.; Shi, T. Z.; Wu, X. W.; Xue, J. Y.; Li, Q. X.; Hua, R. M. J. Agric. Food Chem. 2018, 66, 1023.
doi: 10.1021/acs.jafc.7b05491 |
| [17] |
Neumann, D. M.; Cammarata, A.; Backes, G.; Palmer, G. E.; Jursic, B. S. Bioorg. Med. Chem. 2014, 22, 813.
doi: 10.1016/j.bmc.2013.12.010 |
| [18] |
Guo, H. Y.; Jin, C. M.; Zhang, H. M.; Jin, C.-M.; Shen, Q. K.; Quan, Z. S. J. Agric. Food Chem. 2019, 67, 9630.
doi: 10.1021/acs.jafc.9b02173 |
| [19] |
Palwinder Singh, S. Eur. J. Med. Chem. 2014, 74, 440.
doi: 10.1016/j.ejmech.2013.12.047 |
| [20] |
Singh, P.; Kaur, M.; Verma, P. Bioorg. Med. Chem. Lett. 2009, 19, 3054.
|
| [21] |
Siddiqui, Z. N.; Musthafa, T. N. M.; Ahmad, A.; Khan, A. U. Bioorg. Med. Chem. Lett. 2011, 21, 2860.
doi: 10.1016/j.bmcl.2011.03.080 |
| [22] |
Yan, Q.; Cao, R. H.; Yi, W.; Chen, Z. Y.; Wen, H.; Ma, L.; Song, H. C. Eur. J. Med. Chem. 2009, 44, 4235.
doi: 10.1016/j.ejmech.2009.05.023 |
| [23] |
Neumann, D. M.; Cammarata, A.; Backes, G.; Palmer, G. E.; Jursic, B. S. Bioorg. Med. Chem. 2014, 22, 813.
doi: 10.1016/j.bmc.2013.12.010 |
| [24] |
Gao, Y.; Xie, J. S.; Tang, R. T.; Yang, K. Y.; Zhang, Y. H.; Chen, L. X.; Li, H. Bioorg. Chem. 2019, 85, 168.
doi: 10.1016/j.bioorg.2018.12.018 |
| [25] |
Laxmi, S. V.; Reddy, Y. T.; Kuarm, B. S.; Reddy, P. N.; Crooks, P. A.; Rajitha, B. Bioorg. Med. Chem. Lett. 2011, 21, 4329.
doi: 10.1016/j.bmcl.2011.05.055 |
| [26] |
Freeman-Cook, K. D.; Reiter, L. A.; Noe, M. C.; Antipas, A. S.; Danley, D. E.; Datta, K.; Downs, J. T.; Eisenbeis, S.; Eskra, J. D.; Garmene, D. J.; Greer, E. M.; Griffiths, R. J.; Guzman, R.; Hardink, J. R.; Janat, F.; Jones, C. S.; Martinelli, G. J.; Mitchell, P. G.; Laird, E. R.; Liras, J. L.; Lopresti-Morrow, L. L.; Pandit, J.; Reilly, U. D.; Robertson, D.; Vaughn-Bowser, M. L.; Wolf-Gouviea, L. A.; Yocum, S. A. Bioorg. Med. Chem. Lett. 2007, 17, 6529.
doi: 10.1016/j.bmcl.2007.09.085 |
| [27] |
Kim, S. H.; Pudzianowski, A. T.; Leavitt, K. J.; Barbosa, J.; McDonnell, P. A.; Metzler, W. J.; Rankin, B. M.; Liu, R.; Vaccaro, W.; Pitts, W. Bioorg. Med. Chem. Lett. 2005, 15, 1101.
doi: 10.1016/j.bmcl.2004.12.016 |
| [28] |
Guerin, D. J.; Mazeas, D.; Musale, M. S.; Naguib, F. N. M.; Safarjalani, O. N. A.; Kouni, M. H.; Panzica, R. P. Bioorg. Med. Chem. Lett. 1999, 9, 1477.
pmid: 10386920 |
| [29] |
Cordato, D. J.; Herkes, G. K.; Mather, L. E.; Morgan, M. K. J. Clin. Neurosci. 2003, 10, 283.
pmid: 12763328 |
| [30] |
Willow, M.; Johnston, G. A. R. Int. Rev. Neurobiol. 1983, 24, 15.
pmid: 6140244 |
| [31] |
Lee, D. L.; Carter, C. G. US 4797147A, 1989.
|
| [32] |
Lei, K.; Li, P.; Zhou, X. Y.; Wang, S. B.; Wang, X. K.; Ji, L. S.; Liu, R. M.; Xu, X. H. Chin. J. Org. Chem. 2020, 40, 2788. (in Chinese).
doi: 10.6023/cjoc202005031 |
|
(雷康, 李攀, 周晓芸, 王世本, 王学堃, 冀卢莎, 柳仁民, 徐效华, 有机化学, 2020, 40, 2788.)
doi: 10.6023/cjoc202005031 |
|
| [33] |
Lei, K.; Li, P.; Yang, X. F.; Wang, S. B.; Wang, X. K.; Hua, X.W. Sun, B.; Ji, L. S.; Xu, X. H. J. Agric. Food Chem. 2019, 67, 10489.
doi: 10.1021/acs.jafc.9b03109 |
| [34] |
Lei, K.; Liu, Y.; Wang, S. B.; Sun, B.; Hua, X. W.; Xu, X. H. ; Chem. Res. Chin. Univ. 2019, 35, 609.
doi: 10.1007/s40242-019-9029-1 |
| [35] |
Li, N.; Fu, L. L.; Wang, S. B.; Liu, Y.; Lei, K.; Xu, X. H. J. Liaocheng Univ. (Nat. Sci. Ed.) 2018, 31, 53. (in Chinese).
|
|
(李娜, 付琳琳, 王世本, 刘洋, 雷康, 徐效华, 聊城大学学报(自然科学版), 2018, 31, 53.)
|
|
| [36] |
Lei, K.; Hua, X. W.; Tao, Y.-Y.; Liu, Y.; Liu, N.; Ma, Y.; Li, Y. H.; Xu, X. H.; Kong, C. H. Bioorg. Med. Chem. 2016, 24, 92.
doi: 10.1016/j.bmc.2015.11.032 |
| [1] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [2] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [3] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [4] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [5] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [6] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [7] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [8] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [9] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [10] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [11] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [12] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [13] | Ruixia Cao, Yuping Jia. Synthesis and Biological Activity of Novel Pyrrolo[2,3-d]pyrimidine Derivatives Containing Coumarin [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3304-3311. |
| [14] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [15] | Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||