Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (8): 3272-3278.DOI: 10.6023/cjoc202103054 Previous Articles Next Articles
ARTICLES
收稿日期:2021-03-28
修回日期:2021-04-25
发布日期:2021-05-08
通讯作者:
夏远志
基金资助:Received:2021-03-28
Revised:2021-04-25
Published:2021-05-08
Contact:
Yuanzhi Xia
Supported by:Share
Man Xu, Yuanzhi Xia. Mechanistic Understanding of Rh(III)-Catalyzed Redox-Neutral C—H Activation/Annulation Reactions of N-Phenoxyacetamides and Methyleneoxetanones[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3272-3278.

| [1] |
(a) Geepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192.
doi: 10.1021/acs.chemrev.8b00507 pmid: 28530807 |
|
(b) Xue, X.-S.; Ji, P.; Zhou, B.; Cheng, J.-P. Chem. Rev. 2017, 117, 8622.
doi: 10.1021/acs.chemrev.6b00664 pmid: 28530807 |
|
|
(c) Davies, D. L.; Macgregor, S. A.; McMullin, C. L. Chem. Rev. 2017, 117, 8649.
doi: 10.1021/acs.chemrev.6b00839 pmid: 28530807 |
|
| [2] |
(a) Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007.
doi: 10.1021/acs.accounts.5b00077 pmid: 27072661 |
|
(b) Wang, R.; Xie, X.; Liu, H.; Zhou, Y. Catalysts 2019, 9, 823.
doi: 10.3390/catal9100823 pmid: 27072661 |
|
|
(c) Gensch, T.; Hopkinson, M. N.; Glorius, F.; Wencel-Delord, J. Chem. Soc. Rev. 2016, 45, 2900.
doi: 10.1039/c6cs00075d pmid: 27072661 |
|
|
(d) Song, G.; Wang, F.; Li, X. Chem. Soc. Rev. 2012, 41, 3651.
doi: 10.1039/c2cs15281a pmid: 27072661 |
|
| [3] |
(a) Shin, K.; Kim, H.; Chang, S. Acc. Chem. Res. 2015, 48, 1040.
doi: 10.1021/acs.accounts.5b00020 |
|
(b) Huang, H.; Ji, X.; Wu, W.; Jiang, H. Chem. Soc. Rev. 2015, 44, 1155.
doi: 10.1039/C4CS00288A |
|
|
(c) Huang, H.; Cai, J.; Deng, G.-J. Org. Biomol. Chem. 2016, 14, 1519.
doi: 10.1039/C5OB02417J |
|
|
(d) Mo, J.; Wang, L.; Liu, Y.; Cui, X. Synthesis 2015, 47, 439.
doi: 10.1055/s-00000084 |
|
|
(e) Hu, Z.; Tong, X.; Liu, G. Chin. J. Org. Chem. 2015, 35, 539. (in Chinese)
doi: 10.6023/cjoc201412050 |
|
|
(胡志勇, 童晓峰, 刘桂霞, 有机化学, 2015, 35, 539.)
doi: 10.6023/cjoc201412050 |
|
| [4] |
(a) Patureau, F. W.; Glorius, F. Angew. Chem., Int. Ed. 2011, 50, 1977.
doi: 10.1002/anie.v50.9 pmid: 21275421 |
|
(b) Rakshit, S.; Grohmann, C.; Besset, T.; Glorius, F. J. Am. Chem. Soc. 2011, 133, 2350.
doi: 10.1021/ja109676d pmid: 21275421 |
|
|
(c) Guimond, N.; Gorelsky, S. I.; Fagnou, K. J. Am. Chem. Soc. 2011, 133, 6449.
doi: 10.1021/ja201143v pmid: 21275421 |
|
|
(d) Too, P. C.; Noji, T.; Lim, Y. J.; Li, X.; Chiba, S. Synlett 2011, 2789.
pmid: 21275421 |
|
| [5] |
(a) Pan, J.-L.; Liu, C.; Chen, C.; Liu, T.-Q.; Wang, M.; Sun, Z.; Zhang, S.-Y. Org. Lett. 2019, 21, 2823.
doi: 10.1021/acs.orglett.9b00812 |
|
(b) Wang, S.-B.; Zheng, J.; You, S.-L. Org. Lett. 2018, 20, 7131.
doi: 10.1021/acs.orglett.8b03082 |
|
|
(c) Pan, J.-L.; Xie, P.; Chen, C.; Hao, Y.; Liu, C.; Bai, H.-Y.; Ding, J.; Wang, L.-R.; Xia, Y.; Zhang, S.-Y. Org. Lett. 2018, 20, 7131.
doi: 10.1021/acs.orglett.8b03082 |
|
|
(d) Li, X. G.; Liu, K.; Zou, G.; Liu, P. N. Adv. Synth. Catal. 2014, 356, 1496.
doi: 10.1002/adsc.v356.7 |
|
|
(e) Huang, X.; Huang, J.; Du, C.; Zhang, X.; Song, F.; You, J. Angew. Chem.,Int. Ed. 2013, 52, 12970.
doi: 10.1002/anie.201307174 |
|
|
(f) Zheng, L.; Hua, R. Chem.-Eur. J. 2014, 20, 2352.
doi: 10.1002/chem.201304302 |
|
|
(g) Too, P. C.; Wang, Y.-F.; Chiba, S. Org. Lett. 2010, 12, 5688.
doi: 10.1021/ol102504b |
|
|
(h) Krieger, J.-P.; Lesuisse, D.; Ricci, G.; Perrin, M.-A.; Meyer, C.; Cossy, J. Org. Lett. 2017, 19, 2706.
doi: 10.1021/acs.orglett.7b01051 |
|
|
(i) Hu, Z.; Tong, X.; Liu, G. Org. Lett. 2016, 18, 1702.
doi: 10.1021/acs.orglett.6b00616 |
|
|
(j) Semakul, N.; Jackson, K. E.; Paton, R. S.; Rovis, T. Chem. Sci. 2017, 8, 1015.
doi: 10.1039/C6SC02587K |
|
|
(k) Wu, S.; Zeng, R.; Fu, C.; Yu, Y.; Zhang, X.; Ma, S. Chem. Sci. 2015, 6, 2275.
doi: 10.1039/C5SC00092K |
|
|
(l) Wang, Y.; Chen, Y.; Yang, Y.; Zhou, B. Org. Chem. Front. 2018, 5, 1844.
doi: 10.1039/C8QO00265G |
|
|
(m) Wu, J.; Cui, X.; Chen, L.; Jiang, G.; Wu, Y. J. Am. Chem. Soc. 2009, 131, 13888.
doi: 10.1021/ja902762a |
|
| [6] |
(a) Liu, B.; Song, C.; Sun, C.; Zhou, S.; Zhu, J. J. Am. Chem. Soc. 2013, 135, 16625.
doi: 10.1021/ja408541c |
|
(b) Liu, B.; Song, C.; Sun, C.; Zhou, S.; Zhu, J. J. Am. Chem. Soc. 2013, 135, 16625.
doi: 10.1021/ja408541c |
|
|
(c) Muralirajan, K.; Haridharan, R.; Prakash, S.; Cheng, C.-H. Adv. Synth. Catal. 2015, 357, 761.
doi: 10.1002/adsc.v357.4 |
|
| [7] |
(a) Mo, J.; Wang, L.; Cui, X. Org. Lett. 2015, 17, 4960.
doi: 10.1021/acs.orglett.5b02291 |
|
(b) Yu, S.; Liu, S.; Lan, Y.; Wan, B.; Li, X. J. Am. Chem. Soc. 2015, 137, 1623.
doi: 10.1021/ja511796h |
|
|
(c) Wang, P.; Xu, Y.; Sun, J.; Li, X. Org. Lett. 2019, 21, 8459.
doi: 10.1021/acs.orglett.9b03226 |
|
|
(d) Wang, Q.; Li, Y.; Qi, Z.; Xie, F.; Lan, Y.; Li, X. ACS Catal. 2016, 6, 1971.
doi: 10.1021/acscatal.5b02297 |
|
| [8] |
(a) Liu, S.; Pu, M.; Wu, Y.-D.; Zhang, X. J. Org. Chem. 2020, 85, 12594.
doi: 10.1021/acs.joc.0c01775 pmid: 26951887 |
|
(b) Hu, W.; Li, J.; Xu, Y.; Li, J.; Wu, W.; Liu, H.; Jiang, H. Org. Lett. 2017, 19, 678.
doi: 10.1021/acs.orglett.6b03852 pmid: 26951887 |
|
|
(c) Zhu, Z.; Tang, X.; Li, X.; Wu, W.; Deng, G.; Jiang, H. J. Org. Chem. 2016, 81, 1401.
doi: 10.1021/acs.joc.5b02376 pmid: 26951887 |
|
|
(d) Li, X.-C.; Du, C.; Zhang, H.; Niu, J.-L.; Song, M.-P. Org. Lett. 2019, 21, 2863.
doi: 10.1021/acs.orglett.9b00866 pmid: 26951887 |
|
|
(e) Muralirajan, K.; Kuppusamy, R.; Prakash, S.; Chenga, C.-H. Adv. Synth. Catal. 2016, 358, 774.
doi: 10.1002/adsc.201501056 pmid: 26951887 |
|
|
(f) Zhu, C.; Zhu, R.; Zeng, H.; Chen, F.; Liu, C.; Wu, W.; Jiang, H. Angew. Chem., Int. Ed. 2017, 56, 13324.
doi: 10.1002/anie.201707719 pmid: 26951887 |
|
|
(g) Sivakumar, G.; Vijeta, A.; Jeganmohan, M. Chem.-Eur. J. 2016, 22, 5899.
doi: 10.1002/chem.201600471 pmid: 26951887 |
|
|
(h) Sen, M.; Mandal, R.; Das, A.; Kalsi, D.; Sundararaju, B. Chem.- Eur. J. 2017, 23, 17454.
doi: 10.1002/chem.v23.69 pmid: 26951887 |
|
|
(i) Zhou, S.; Wang, J.; Chen, P.; Chen, K.; Zhu, J. Chem.-Eur. J. 2016, 22, 14508.
doi: 10.1002/chem.201602936 pmid: 26951887 |
|
|
(j) Yu, X.; Chen, K.; Guo, S.; Shi, P.; Song, C. Zhu, J. Org. Lett. 2017, 19, 5348.
doi: 10.1021/acs.orglett.7b02632 pmid: 26951887 |
|
|
(k) Jiang, X.; Hao, J.; Zhou, G.; Hou, C.; Hu, F. Chin. J. Org. Chem. 2019, 39, 1811.
pmid: 26951887 |
|
|
(l) Wang, Z.; Xie, P.; Xia, Y. Chin. Chem. Lett. 2018, 29, 47.
doi: 10.1016/j.cclet.2017.06.018 pmid: 26951887 |
|
|
(m) Muniraj, N.; Prabhu, K. R. Org. Lett. 2019, 21, 1068.
doi: 10.1021/acs.orglett.8b04117 pmid: 26951887 |
|
|
(n) Yu, C.; Li, F.; Zhang, J.; Zhong, G. Chem. Commun. 2017, 53, 533.
doi: 10.1039/C6CC07064G pmid: 26951887 |
|
|
(o) Kaishap, P. P.; Sarmab, B.; Gogoi, S. Chem. Commun. 2016, 52, 9809.
doi: 10.1039/C6CC04461A pmid: 26951887 |
|
|
(p) Zhou, J.; Shi, J.; Qi, Z.; Li, X.; Xu, H. E.; Yi, W. ACS Catal. 2015, 5, 6999.
doi: 10.1021/acscatal.5b01571 pmid: 26951887 |
|
|
(q) Petrova, E.; Rasina, D.; Jirgensons, A. Eur. J. Org. Chem. 2017, 2017, 1773.
doi: 10.1002/ejoc.v2017.13 pmid: 26951887 |
|
| [9] |
(a) Xu, L.; Zhu, Q.; Huang, G.; Cheng, B.; Xia, Y. J. Org. Chem. 2012, 77, 3017.
doi: 10.1021/jo202431q pmid: 25982708 |
|
(b) Guo, W.; Zhou, T.; Xia, Y. Organometallics 2015, 34, 3012.
doi: 10.1021/acs.organomet.5b00317 pmid: 25982708 |
|
|
(c) Zhou, T.; Guo, W.; Xia, Y. Chem.-Eur. J. 2015, 21, 9209.
doi: 10.1002/chem.201500558 pmid: 25982708 |
|
| [10] |
Vásquez-Céspedes, S.; Wang, X.; Glorius, F. ACS Catal. 2018, 8, 242.
doi: 10.1021/acscatal.7b03048 |
| [11] |
(a) Liu, S.; Qi, X.; Qu, L.-B.; Bai, R.; Lan, Y. Catal. Sci. Technol. 2018, 8, 1645.
doi: 10.1039/C7CY02367G pmid: 27997056 |
|
(b) Li, Y.; Chen, H.; Qu, L.-B.; Houk, K. N.; Lan, Y. ACS Catal. 2019, 9, 7154.
doi: 10.1021/acscatal.9b02085 pmid: 27997056 |
|
|
(c) Funes-Ardoiz, I.; Maseras, F. ACS Catal. 2018, 8, 1161.
doi: 10.1021/acscatal.7b02974 pmid: 27997056 |
|
|
(d) Wu, W.; Ren, D.; Xu, B.; Ma, X.; Huang, C.; Zhang, J.; Liu, T. Asian J. Org. Chem. 2017, 6, 1885.
doi: 10.1002/ajoc.201700469 pmid: 27997056 |
|
|
(e) Wu, J.-Q.; Zhang, S.-S.; Gao, H.; Qi, Z.; Zhou, C.-J.; Ji, W.-W.; Liu, Y.; Chen, Y.; Li, Q.; Li, X.; Wang, H. J. Am. Chem. Soc. 2017, 139, 3537.
doi: 10.1021/jacs.7b00118 pmid: 27997056 |
|
|
(f) Xing, Y.-Y.; Liu, J.-B.; Sheng, X.-H.; Sun, C.-Z.; Huang, F.; Chen, D.-Z. Inorg. Chem. 2017, 56, 5392.
doi: 10.1021/acs.inorgchem.7b00450 pmid: 27997056 |
|
|
(g) Xing, Z.; Huang, F.; Sun, C.; Zhao, X.; Liu, J.; Chen, D. Inorg. Chem. 2015, 54, 3958.
doi: 10.1021/acs.inorgchem.5b00134 pmid: 27997056 |
|
|
(h) Zhang, T.; Qi, X.; Liu, S.; Bai, R.; Liu, C.; Lan, Y. Chem.-Eur. J. 2017, 23, 2690.
doi: 10.1002/chem.201605188 pmid: 27997056 |
|
|
(i) Du, L.; Xu, Y.; Yang, S.; Li, J.; Fu, X. J. Org. Chem. 2016, 81, 1921.
doi: 10.1021/acs.joc.5b02747 pmid: 27997056 |
|
| [12] |
(a) Wang, X.; Gensch, T.; Lerchen, A.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 6506.
doi: 10.1021/jacs.7b02725 |
|
(b) Yang, Y.-F.; Houk, K. N.; Wu, Y.-D. J. Am. Chem. Soc. 2016, 138, 6861.
doi: 10.1021/jacs.6b03424 |
|
|
(c) Wu, Y.; Chen, Z.; Yang, Y.; Zhu, W.; Zhou, B. J. Am. Chem. Soc. 2018, 140, 42.
doi: 10.1021/jacs.7b10349 |
|
|
(d) Qiu, Z.; Deng, J.; Zhang, Z.; Wu, C.; Li, J.; Liao, X. Dalton Trans. 2016, 45, 8118.
doi: 10.1039/C6DT00093B |
|
| [13] |
(a) Ling, B.; Liu, Y.; Jiang, Y.-Y.; Liu, P.; Bi, S. Organometallics 2019, 38, 1877.
doi: 10.1021/acs.organomet.8b00769 |
|
(b) Lau, S.; Ward, B.; Zhou, X.; White, A. J. P.; Casely, I. J.; Macgregor, S. A.; Crimmin, M. R. Organometallics 2017, 36, 3654.
doi: 10.1021/acs.organomet.7b00632 |
|
|
(c) Song, L.; Zhang, X.; Tang, X.; Meervelt, L. V.; Eycken, J. V. D.; Harvey, J. N.; Eycken, E. V. V. D. Chem. Sci. 2020, 11, 11562.
doi: 10.1039/D0SC04434B |
|
|
(d) Ling, B.; Wang, J.; Liu, Y.; Jiang, Y.-Y.; Liu, P.; Feng, J.; Bi, S. Eur. J. Org. Chem. 2021, 2021, 266.
doi: 10.1002/ejoc.v2021.2 |
|
|
(e) Lian, B.; Zhang, L.; Fang, D.-C. Org. Chem. Front. 2019, 6, 2600.
doi: 10.1039/C9QO00154A |
|
|
(f) Rogge, T.; Ackermann, L. Angew. Chem., Int. Ed. 2019, 58, 15640.
doi: 10.1002/anie.v58.44 |
|
| [14] |
(a) Li, S.; Shi, P.; Liu, R.-H.; Hu, X.-H.; Loh, T.-P. Org. Lett. 2019, 21, 1602.
doi: 10.1021/acs.orglett.9b00141 |
|
(b) Xing, Y.-Y.; Liu, J.-B.; Sun, C.-Z.; Huang, F.; Chen, D.-Z. Inorg. Chem. 2018, 57, 10726.
doi: 10.1021/acs.inorgchem.8b01352 |
|
| [15] |
(a) Liu, S.; Pu, M.; Wu, Y.-D.; Zhang, X. J. Org. Chem. 2020, 85, 12594.
doi: 10.1021/acs.joc.0c01775 pmid: 30668096 |
|
(b) Zhang, M.; Liang, J.; Huang, G. J. Org. Chem. 2019, 84, 2372.
doi: 10.1021/acs.joc.9b00117 pmid: 30668096 |
|
|
(c) Zhu, L.; Qi, X.; Li, Y.; Duan, M.; Zou, L.; Bai, R.; Lan, Y. Organometallics 2017, 36, 2107.
doi: 10.1021/acs.organomet.7b00151 pmid: 30668096 |
|
|
(d) Erbing, E.; Sanz-Marco, A.; Vázquez-Romero, A.; Malmberg, J.; Johansson, M. J.; Gómez-Bengoa, E.; Martín-Matute, B. ACS Catal. 2018, 8, 920.
doi: 10.1021/acscatal.7b02987 pmid: 30668096 |
|
|
(e) Gao, P.; Guo, W.; Xue, J.; Zhao, Y.; Yuan, Y.; Xia, Y.; Shi, Z. J. Am. Chem. Soc. 2015, 137, 12231.
doi: 10.1021/jacs.5b06758 pmid: 30668096 |
|
|
(f) Park, Y.; Heo, J.; Baik, M.-H.; Chang, S. J. Am. Chem. Soc. 2016, 138, 14020.
doi: 10.1021/jacs.6b08211 pmid: 30668096 |
|
| [16] |
Zhou, Z.; Bian, M.; Zhao, L.; Gao, H.; Huang, J.; Liu, X.; Yu, X.; Li, X.; Yi, W. Org. Lett. 2018, 20, 3892.
doi: 10.1021/acs.orglett.8b01477 pmid: 29897772 |
| [17] |
Wu, H.; Li, X.; Tang, X.; Feng, C.; Huang, G. J. Org. Chem. 2018, 83, 9220.
doi: 10.1021/acs.joc.8b01229 |
| [18] |
(a) Chen, W.-J.; Lin, Z. Organometallics 2015, 34, 309.
doi: 10.1021/om501130c |
|
(b) Guo, W.; Xia, Y. J. Org. Chem. 2015, 80, 8113.
doi: 10.1021/acs.joc.5b01201 |
|
|
(c) Chen, J.; Guo, W.; Xia, Y. J. Org. Chem. 2016, 81, 2635.
doi: 10.1021/acs.joc.6b00003 |
|
| [19] |
(a) Jiang, Y.-Y.; Man, X.; Bi, S. Sci. China: Chem. 2016, 59, 1448.
pmid: 24815788 |
|
(b) Liu, L. L.; Wu, Y.; Wang, T.; Gao, X.; Zhu, J.; Zhao, Y. J. Org. Chem. 2014, 79, 5074.
doi: 10.1021/jo500616g pmid: 24815788 |
|
| [20] |
(a) Ma, X.-X.; Liu, J.-B.; Huang, F.; Sun, C.-Z.; Chen, D.-Z. Catal. Sci. Technol. 2018, 8, 3590.
doi: 10.1039/C8CY00481A pmid: 26197041 |
|
(b) Ramesh, B.; Tamizmani, M.; Jeganmohan, M. J. Org. Chem. 2019, 84, 4058.
doi: 10.1021/acs.joc.9b00051 pmid: 26197041 |
|
|
(c) Wu, W.; Liu, Y.; Bi, S. Org. Biomol. Chem. 2015, 13, 8251.
doi: 10.1039/C5OB00977D pmid: 26197041 |
|
|
(d) Wu, S.; Huang, X.; Wu, W.; Li, P.; Fu, C.; Ma, S. Nat. Commun. 2015, 6, 7946.
doi: 10.1038/ncomms8946 pmid: 26197041 |
|
|
(e) Neufeldt, S. R.; Jiménez-Osés, G.; Huckins, J. R.; Thiel, O. R.; Houk, K. N. J. Am. Chem. Soc. 2015, 137, 9843.
doi: 10.1021/jacs.5b03535 pmid: 26197041 |
|
| [21] |
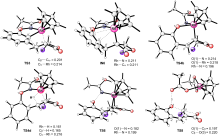
The reaction between 1a and 2a was preliminarily studied by the DFT method by Yi et al. (Ref. [16]), however, a high energy conformation was applied in the olefin insertion and following steps, and the β-elimination pathway was not considered from the olefin insertion intermediate. The reported energies in literature are not consistent with the experimental results.
|
| [22] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A.; Peralta, Jr. J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford CT, 2009.
|
| [23] |
(a) Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
doi: 10.1063/1.464913 pmid: 9944570 |
|
(b) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785.
pmid: 9944570 |
|
| [24] |
(a) Ditchfield, R.; Hehre, W. J.; Pople, J. A. J. Chem. Phys. 1971, 54, 724.
doi: 10.1063/1.1674902 |
|
(b) Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213.
doi: 10.1007/BF00533485 |
|
|
(c) Hehre, W. J.; Ditchfield, R.; Pople, J. A. J. Chem. Phys. 1972, 56, 2257.
doi: 10.1063/1.1677527 |
|
| [25] |
(a) Cruz, V. L.; Muñoz-Escalona, A.; Martinez-Salazar, J. Polymer 1996, 37, 1663.
doi: 10.1016/0032-3861(96)83716-5 |
|
(b) Ehlers, A. W.; Böhme, M.; Dapprich, S.; Gobbi, A.; Höllwarth, A.; Jonas, V.; Köhler, K. F.; Stegmann, R.; Veldkamp, A.; Frenking, G. Chem. Phys. Lett. 1993, 208, 111.
doi: 10.1016/0009-2614(93)80086-5 |
|
|
(c) Roy, L. E.; Hay, P. J.; Martin, R. L. J. Chem. Theory Comput. 2008, 4, 1029.
|
|
| [26] |
Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157.
doi: 10.1021/ar700111a |
| [27] |
(a) Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Theor. Chim. Acta 1990, 77, 123.
doi: 10.1007/BF01114537 |
|
(b) Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866.
doi: 10.1063/1.452288 |
|
| [28] |
Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650.
|
| [1] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [2] | Cunjing Miao, Jiaqi Yao. Recent Advances in the Transformation Reactions of Aromatic Nitriles via C—CN Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1341-1364. |
| [3] | Tingting Liu, Yucai Hu, An Shen. Mechanism of Carbon-Carbon Coupling Reactions Catalyzed by Imine-Ligand-Assisted N-Heterocyclic Carbene Palladium Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 622-628. |
| [4] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [5] | Fei Chen, Sheng Tao, Ning Liu, Bin Dai. CNN-Type Binuclear Cu(I) Complexes Catalyzed Direct Carboxylation via the Fixation of CO2 at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2471-2480. |
| [6] | Zexin Huang, Yuqiang Yin, Fengcheng Jia, Anxin Wu. Research Progress on C2—C3 Bond Cleavage of Indole and Its Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2028-2044. |
| [7] | Cheng Gong, Jian Tang, Fei Xu, Pengjie Li, Zetian Wang, Yumin Zhang, Guoxian Yu, Liang Wang. Recent Advances in Transition-Metal-Catalyzed C—H Activation of Pyridone/Isoquinolones [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1925-1949. |
| [8] | Chengyao Kimmy Cao, Yaru Niu, Yunchen Jiang, Hongmei Qu, Chao Chen. Pd-Catalyzed Chlorodifluoroethylation Reaction of C—H Bond of Acetanilides with Chlorodifluoroethyl Iodonium Reagents [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2098-2105. |
| [9] | Chaoyu Wang, Shuda Dong, Tianyang Zhu, Yuqin Liu, Zihan Wu, Ruokun Feng. Cobalt-Catalyzed Decarbonylative C(8)-Acyloxylation of 1-Naphthalamine Derivatives with α-Oxocarboxylic Acids [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1799-1810. |
| [10] | Yubing Shi, Wenji Bai, Weihua Mu, Jiangping Li, Jiawei Yu, Bing Lian. Research Progress on Density Functional Theory Study of Palladium-Catalyzed C—H Functionalization to Form C—X (X=O, N, F, I, …) Bonds [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1346-1374. |
| [11] | Xinyao Wang, Qingqing Zhang, Shuyang Liu, Min Li, Haifang Li, Chunying Duan, Yunhe Jin. Visible Light-Induced Metal-Free Benzylation of Quinones via Cross Dehydrogenation Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1443-1452. |
| [12] | Youcai Zhu, Xinxin Ding, Li Sun, Zhen Liu. Advances in the Production of Acrylic Acid and Its Derivatives by CO2/C2H4 Coupling [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 965-977. |
| [13] | Zheng Li, Yingchun Gu, Dazhen Xu, Xuening Fei, Lei Zhang. Density Functional Theory Study on the Mechanism of Organophosphine-Catalyzed [4+2] Cycloaddition Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 830-837. |
| [14] | Yudong Li, Ying Li, Ya'nan Dong, Chungu Xia, Yuehui Li. Manganese-Catalyzed Allylation of Quinazolinones with 4-Vinyl-1,3-dioxolan-2-one via C—H Activation [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 847-853. |
| [15] | Lingjie Fan, Tao Zhou, Xu Yang, Mengxue Jiang, Xinquan Hu, Bingfeng Shi. Pd(II)-Catalyzed Enantioselective C—H Olefination of 2-(Arylsulfinyl)pyridines through Kinetic Resolution [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3405-3418. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
