化学学报 ›› 2019, Vol. 77 ›› Issue (10): 1045-1053.DOI: 10.6023/A19060205 上一篇 下一篇
研究论文
投稿日期:2019-06-10
发布日期:2019-08-28
通讯作者:
杨洋
E-mail:yyang@phy.ecnu.edu.cn
基金资助:
Yang, Penglia, Wang, Zhenxingb, Liang, Zuna, Liang, Hongtaoa, Yang, Yanga*( )
)
Received:2019-06-10
Published:2019-08-28
Contact:
Yang, Yang
E-mail:yyang@phy.ecnu.edu.cn
Supported by:文章分享
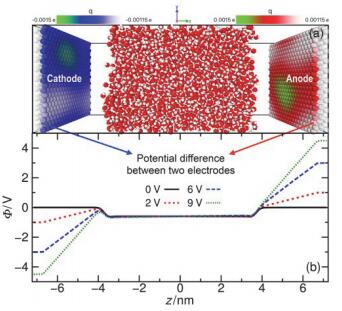
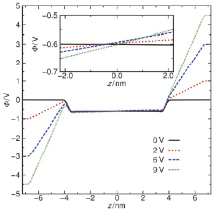
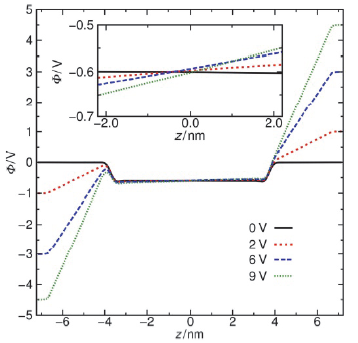
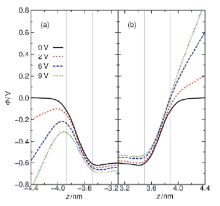
水表面电势在诸多电化学过程与反应中扮演关键角色, 然而实验上直接测量却极具挑战. 本论文提出一套基于平衡态恒定电势分子动力学的模拟-分析-计算方法, 可实现通过保持恒定电势且伴随电荷涨落的电极板将电场作用于附近的水表面, 并以平均探针电势计算方法精确测量空间电势分布. 凭借此套方法, 首次计算了不同电极电势下水表面区域的空间电势分布函数, 并测得了鲜有报道的水表面电势随外电场的变化关系. 发现了阴极附近水的表面电势随外电场增强而降低而阳极附近水的表面电势随外电场增强而增大的非对称性. 同时计算了平衡态水表面分子数密度和偶极矩极化密度分布函数, 展示出逐渐增强的外电场能够强烈改变水表面区域的极化行为也能够使液体水整体微弱的极化. 论文最后提出水表面电势随电场变化的非对称性源自水表面极化行为的非对称性以及液体区域的整体极化.
杨鹏里, 王振兴, 梁尊, 梁洪涛, 杨洋. 电场作用下水表面电势的分子动力学研究[J]. 化学学报, 2019, 77(10): 1045-1053.
Yang, Pengli, Wang, Zhenxing, Liang, Zun, Liang, Hongtao, Yang, Yang. A Molecular Dynamics Simulation Study of the Effect of External Electric Field on the Water Surface Potential[J]. Acta Chimica Sinica, 2019, 77(10): 1045-1053.
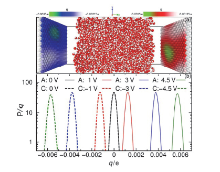
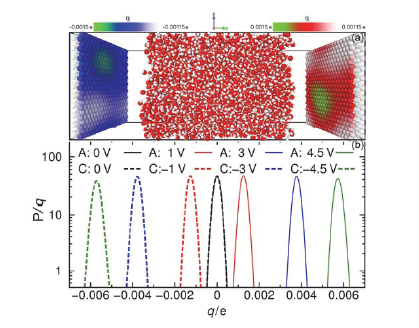
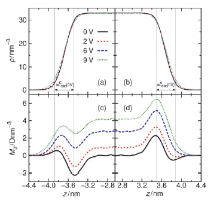

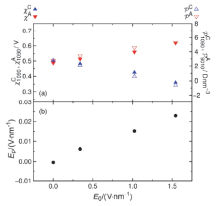
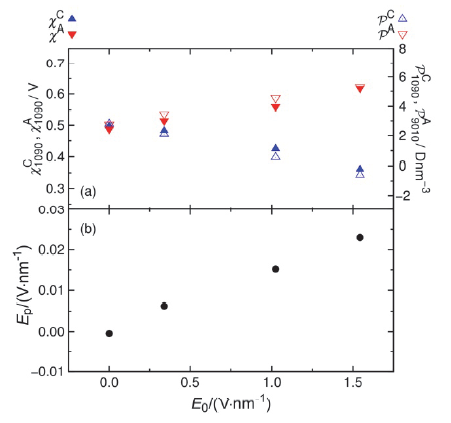
| Δ?/V | $w_{1090}^{C}\text{/nm}$ | $w_{1090}^{A}\text{/nm}$ | $\chi _{1090}^{C}\text{/V}$ | $\chi _{1090}^{A}\text{/V}$ | (Debye?nm–3) | E0/(V?nm–1) | Ep/(V?nm–1) | |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.389(7) | 0.388(6) | 0.49(1) | 0.49(1) | 2.78(3) | 2.77(3) | 0.00 | 0.000 |
| 2 | 0.393(10) | 0.385(6) | 0.48(2) | 0.51(2) | 2.10(4) | 3.46(5) | 0.34 | 0.006 |
| 6 | 0.421(7) | 0.398(7) | 0.43(3) | 0.56(1) | 0.56(5) | 4.58(3) | 1.02 | 0.015 |
| 9 | 0.467(8) | 0.433(12) | 0.36(3) | 0.62(2) | –0.60(3) | 5.31(4) | 1.55 | 0.023 |
| Δ?/V | $w_{1090}^{C}\text{/nm}$ | $w_{1090}^{A}\text{/nm}$ | $\chi _{1090}^{C}\text{/V}$ | $\chi _{1090}^{A}\text{/V}$ | (Debye?nm–3) | E0/(V?nm–1) | Ep/(V?nm–1) | |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.389(7) | 0.388(6) | 0.49(1) | 0.49(1) | 2.78(3) | 2.77(3) | 0.00 | 0.000 |
| 2 | 0.393(10) | 0.385(6) | 0.48(2) | 0.51(2) | 2.10(4) | 3.46(5) | 0.34 | 0.006 |
| 6 | 0.421(7) | 0.398(7) | 0.43(3) | 0.56(1) | 0.56(5) | 4.58(3) | 1.02 | 0.015 |
| 9 | 0.467(8) | 0.433(12) | 0.36(3) | 0.62(2) | –0.60(3) | 5.31(4) | 1.55 | 0.023 |

| [1] |
Bateni, A.; Susnar, S. S.; Amirfazli, A.; Neumann, A. W . Langmuir 2004, 20, 7589.
doi: 10.1021/la0494167 |
| [2] |
Bateni, A.; Laughton, S. J..; Tavana, H.; Susnar, S. S.; Amirfazli, A.; Neumann, A. Colloid Interface Sci.2005,283, 215.
doi: 10.1016/j.jcis.2004.08.134 |
| [3] |
Eggers, J.; Villermaux, E . Rep. Prog. Phys.2008, 71, 036601.
doi: 10.1088/0034-4885/71/3/036601 |
| [4] |
Yan, J. Y.; Patey, G. N. J. Phys. Chem. Lett. 2011, 2, 2555.
doi: 10.1021/jz201113m |
| [5] |
Yan, J. Y.; Patey, G. N . J. Phys. Chem. A 2012, 116, 7057.
doi: 10.1021/jp3039187 |
| [6] |
Yan, J. Y.; Patey, G. N . J. Chem. Phys. 2013, 139, 144501.
doi: 10.1063/1.4824139 |
| [7] |
Yan, J.; Overduin, S. D.; Patey, G. N . J. Chem. Phys. 2014, 141, 074501.
doi: 10.1063/1.4892586 |
| [8] |
Zhang, Z. S.; Liu, X. Y. Chem. Soc. Rev. 2018, 47, 7116.
doi: 10.1039/C8CS00626A |
| [9] | Dash, J. G.; Rempel, A. W.; Wettlaufer, J. S . Rev. Mod. Phys. 2006, 78, 3. |
| [10] |
Qiu, H.; Guo, W. L . Phys. Rev. Lett. 2013, 110, 195701.
doi: 10.1103/PhysRevLett.110.195701 |
| [11] |
Mei, F.; Zhou, X. Y.; Kou, J. L.; Wu, F. M.; Wang, C. L.; Lu, H. J . J. Chem. Phys. 2015,142, 134704.
doi: 10.1063/1.4916521 |
| [12] |
Zangi, R.; Mark, A. E . J. Chem. Phys. 2004, 120, 7123.
doi: 10.1063/1.1687315 |
| [13] |
Choi, E. M.; Yoon, Y. H.; Lee, S.; Kang, H . Phys. Rev. Lett. 2005, 95, 085701.
doi: 10.1103/PhysRevLett.95.085701 |
| [14] |
Ehre, D.; Lavert, E.; Lahav, M.; Lubomirsky, L . Science 2010, 327, 672.
doi: 10.1126/science.1178085 |
| [15] |
Carpenter, K.; Bahadur, V . Langmuir 2015, 31, 2243.
doi: 10.1021/la504792n |
| [16] |
Nandi, P. K.; Burnham, C. J.; English, N. J . J. Chem. Phys. 2018, 148, 044503.
doi: 10.1063/1.5004509 |
| [17] |
Zaragoza, A.; Espinosa, J. R.; Ramos, R.; Cobos, J. A.; Aragones, J. L.; Vega, C.; Sanz, E.; Ramírez, J.; Valeriani, C . J. Phys.: Condens. Mat. 2018, 30, 174002.
doi: 10.1088/1361-648X/aab464 |
| [18] |
Fernández, M. S.; Peeters, F. M.; Neek-Amal, M. Phys. Rev. B 2016, 94, 045436.
doi: 10.1103/PhysRevB.94.045436 |
| [19] |
Vorob’ev, V. S.; Malyshenko, S. P . Phys. Rev. Lett. 2006, 96, 075701.
doi: 10.1103/PhysRevLett.96.075701 |
| [20] |
Maerzke, K. A.; Siepmann, J. I . J. Phys. Chem. B 2010, 114, 4261.
doi: 10.1021/jp9101477 |
| [21] |
Aragones, J. L.; MacDowell, L. G.; Siepmann, J. I.; Vega1, C.Phys. Rev. Lett. 2011, 107, 155702.
doi: 10.1103/PhysRevLett.107.155702 |
| [22] |
Skinnera, L. B.; Benmorea, C. J.; Shyama, B.; J. K. R. Webera, J. K. R; Pariseb, J. B. Proc. Nat. Acad. Sci. U.S.A. 2012, 109, 16463.
doi: 10.1073/pnas.1210732109 |
| [23] |
Saitta, A. M.; Saija, F.; Giaquinta, P. V . Phys. Rev. Lett. 2012, 108, 207801.
doi: 10.1103/PhysRevLett.108.207801 |
| [24] |
Futera, Z.; English, N. J . J. Chem. Phys. 2017, 147, 031102.
doi: 10.1063/1.4994694 |
| [25] | Warshavsky, V. B.; Bykov, T. V.; Zeng, X. C . J. Chem. Phys. 2001, 114, 1. |
| [26] |
Han, G. Z.; Meng, J. J . Continuum Mech. Thermodyn. 2018, 30, 817.
doi: 10.1007/s00161-018-0644-8 |
| [27] | Hayes, C. F . J. Phys. Chem. 1975, 79, 16. |
| [28] |
Pethica, B. A . Langmuir 1998, 14, 3115.
doi: 10.1021/la971142i |
| [29] | Sato, M.; Kudo, N.; Saito, N. IEEE Transactions on Industry Applications 1998, 34, 2. |
| [30] |
Vega, C.; Abascal, J. L . F.Phys. Chem. Chem. Phys. 2011, 13, 19663.
doi: 10.1039/c1cp22168j |
| [31] |
Moore, S. G.; Stevens, M. J.; Grest, G. S . Phys. Rev. E 2015, 91, 022309.
doi: 10.1103/PhysRevE.91.022309 |
| [32] |
Shi, B.; Agnihotri, M. V.; Chen, S. H.; Black, R.; Singer, S. J . J. Chem. Phys. 2016, 144, 164702.
doi: 10.1063/1.4945760 |
| [33] |
Koski, J. P.; Moore, S. G.; Grest, G. S.; Stevens, M. J . Phys. Rev. E 2017, 96, 063106.
doi: 10.1103/PhysRevE.96.063106 |
| [34] |
Nikzad, M.; Azimian, A. R.; Rezaei, M.; Nikzad, S . J. Chem. Phys. 2017, 147, 204701.
doi: 10.1063/1.4985875 |
| [35] | Jackson, J. D . Classical Electrodynamics, 3rd ed., Wiley, Hoboken, NJ, 1999. |
| [36] | Griffiths, D. J . Introduction to Electrodynamics, 3rd ed.: Prentice-Hall, Upper Saddle River, NJ, 1999. |
| [37] |
Fumagalli, L.; Esfandiar, A.; Fabregas, R.; Hu, S.; Ares, P.; Janardanan1, A.; Yang, Q.; Radha, B.; Taniguchi, T.; Watanabe, K.; Gomila, G.; Novoselov, K. S.; Geim, A. K. Science 2018, 360, 1339.
doi: 10.1126/science.aat4191 |
| [38] |
Willard, A. P.; Reed, S. K.; Madden, P. A.; Chandler, D . Faraday Discuss. 2009, 141, 423.
doi: 10.1039/B805544K |
| [39] |
Vatamanu, J.; Borodin, O.; Smith, G. D . J. Am. Chem. Soc. 2010, 132, 14825.
doi: 10.1021/ja104273r |
| [40] |
Merlet, C.; Salanne, M.; Rotenberg, B.; Madden, P. A . J. Phys. Chem. C 2011, 115, 16613.
doi: 10.1021/jp205461g |
| [41] |
Merlet, C.; Rotenberg, B.; Madden, P. A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M . Nat. Mater. 2012, 11, 306.
doi: 10.1038/nmat3260 |
| [42] |
Limmer, D. T.; Merlet, C.; Salanne, M.; Chandler, D.; Madden, P. A.; van Roij, P.; Rotenberg, B. Phys. Rev. Lett. 2013, 111, 106102.
doi: 10.1103/PhysRevLett.111.106102 |
| [43] |
Limmer, D. T.; Willard, A. P.; Madden, P.; Chandler, D . Proc. Nat. Acad. Sci. U.S.A. 2013, 110, 4200.
doi: 10.1073/pnas.1301596110 |
| [44] |
Vatamanu, J.; Vatamanu, M.; Bedrov, D . ACS Nano 2015, 9, 5999.
doi: 10.1021/acsnano.5b00945 |
| [45] |
Vatamanu, J.; Bedrov, D . J. Phys. Chem. Lett. 2015, 6, 3594.
doi: 10.1021/acs.jpclett.5b01199 |
| [46] |
Limmer, D. T.; Willard, A. P.; Madden, P. A.; Chandler, D . J. Phys. Chem. C 2015, 119, 24016.
doi: 10.1021/acs.jpcc.5b08137 |
| [47] | Parsons, R . Modern Aspects of Electrochemistry, Vol. 1, Ed.: Bokris,J. O.-M. London, Butterworths, 1954. |
| [48] | Matsumoto, M.; Kataoka, Y . J. Chem. Phys. 1988, 88, 3233. |
| [49] | Brodskaya, E. N.; Zakharov, V. V . J. Chem. Phys. 1995, 2, 4595. |
| [50] |
Wilson, M. A.; Pohorille, A.; Pratt, L. R . J. Chem. Phys. 1988, 88, 3281.
doi: 10.1063/1.453923 |
| [51] |
Sokhan, V. P.; Tildesley, D. J . Mol. Phys. 1997, 92, 625.
doi: 10.1080/002689797169916 |
| [52] |
Kathmann, S. M.; Kuo, I. W.; Mundy, C. J . J. Am. Chem. Soc. 2008, 130, 16556.
doi: 10.1021/ja802851w |
| [53] |
Harder, E.; Roux, B . J. Chem. Phys. 2008, 129, 234706.
doi: 10.1063/1.3027513 |
| [54] |
Randles, J. E. B . Phys. Chem. Liq. 1977, 7, 107.
doi: 10.1080/00319107708084730 |
| [55] |
Pratt, L. R . J. Phys. Chem. 1992, 96, 25.
doi: 10.1021/j100180a010 |
| [56] |
Barraclough, C. G.; McTigue, P. T.; Ng, Y. L. J. Electroanal. Chem. 1992, 329, 9.
doi: 10.1016/0022-0728(92)80205-I |
| [57] |
Parfenyuk, V. I . Colloid J. 2002, 64, 588.
doi: 10.1023/A:1020614010528 |
| [58] |
Yang, L.; Fishbine, B. H.; Migliori, A.; Pratt, L. R . J. Am. Chem. Soc. 2009, 131, 12373.
doi: 10.1021/ja9044554 |
| [59] |
Yang, L.; Fishbine, B. H.; Migliori, A.; Pratt, L. R . J. Chem. Phys. 2010, 132, 044701.
doi: 10.1063/1.3294560 |
| [60] |
Shim, Y.; Kim, H. J.; Jung, Y . Faraday Discuss. 2012, 154, 249.
doi: 10.1039/c1fd00086a |
| [61] |
Feng, G.; Cummings, P. T . J. Phys. Chem. Lett. 2011, 2, 2859.
doi: 10.1021/jz201312e |
| [62] |
Feng, G.; Li, S.; Atchison, J. S.; Presser, V.; Cummings, P. T . J. Phys. Chem. C 2013, 117, 9178.
doi: 10.1021/jp403547k |
| [63] |
Reed, S. K.; Lanning, O. J.; Madden, P. A . J. Chem. Phys. 2007, 126, 084704.
doi: 10.1063/1.2464084 |
| [64] |
Reed, S. K.; Madden, P. A.; Papadopoulos, A . J. Chem. Phys. 2008, 128, 124701.
doi: 10.1063/1.2844801 |
| [65] |
Gingrich, T. R.; Wilson, M . Chem. Phys. Lett. 2010, 500, 178.
doi: 10.1016/j.cplett.2010.10.010 |
| [66] |
Wang, Z. X.; Yang, Y.; Olmsted, D. L.; Asta, M.; Laird, B. B. J. Chem. Phys. 2014, 141, 184102.
doi: 10.1063/1.4899176 |
| [67] |
Doppenschmidt, A.; Butt, H.-J . Langmuir 2000, 16, 6709.
doi: 10.1021/la990799w |
| [68] |
Pickering, I.; Paleico, M.; Sirkin, Y. A. P.; Scherlis, D. A.; Factorovich, M. H . J. Phys. Chem. B 2018, 122, 4880.
doi: 10.1021/acs.jpcb.8b00784 |
| [69] |
Berendsen, H. J. C.; Grigera, J. R.; Straatsma, T. P. J. Phys. Chem. 1987, 91, 6269.
doi: 10.1021/j100308a038 |
| [70] |
Yeh, I. C.; Berkowitz, M . J. Chem. Phys. 1999, 111, 3155.
doi: 10.1063/1.479595 |
| [71] |
Ciccotti, G.; Ryckaert, J. P . Comput. Phys. Rep. 1986, 4, 346.
doi: 10.1016/0167-7977(86)90022-5 |
| [72] | Alejandre, J.; Chapela, D. J. T. A . J. Chem. Phys. 1995, 120, 15. |
| [73] |
Wang, Z. X.; Olmsted, D. L.; Asta, M.; Laird, B. B . J. Phys. Condens. Matter 2016, 28, 464006.
doi: 10.1088/0953-8984/28/46/464006 |
| [74] | Smith, G ., Numerical Solution of Partial Differential Equations: Finite Difference Methods, Oxford, Clarendon, 1985. |
| [75] |
Sachs, J. N.; Crozier, P. S.; Woolf, T. B . J. Chem. Phys. 2004, 121, 10847.
doi: 10.1063/1.1826056 |
| [76] |
Li, S.; Feng, G.; Cummings, P. T . J. Phys. Condens. Matter 2014, 26, 284106.
doi: 10.1088/0953-8984/26/28/284106 |
| [77] | Skollermo, G . Math. Comput. 1975, 29, 697. |
| [78] |
Yang, Y.; Laird, B. B . J. Phys. Chem. B 2014, 118, 8373.
doi: 10.1021/jp5019313 |
| [79] | Reynolds, W ., Thermodynamic Properties in SI: Graphs, Tables, and Computational Equations for Forty Substances, Stanford, CA, Dept. of Mechanical Engineering, Stanford University, 1979. |
| [80] |
Warshavsky, V.; Zeng, X. C . Phy. Rev. E 2003, 68, 051203
doi: 10.1103/PhysRevE.68.051203 |
| [81] |
Richmond, G. L . Chem. Rev. 2002, 102, 2693.
doi: 10.1021/cr0006876 |
| [1] | 史雨晴, 储名珠, 韩波, 马豪杰, 李然, 侯雪艳, 张玉琦, 王记江. 智能二维光子晶体水凝胶精准检测Hg2+[J]. 化学学报, 2024, 82(1): 9-15. |
| [2] | 张正初, 熊炜, 吕华. α-螺旋聚氨基酸交联的水凝胶的制备和材料特性★[J]. 化学学报, 2023, 81(9): 1113-1119. |
| [3] | 王端达, 沈欣怡, 宋永杨, 王树涛. 新兴Janus颗粒在油水分离中的应用进展★[J]. 化学学报, 2023, 81(9): 1187-1195. |
| [4] | 王海朋, 蔡文生, 邵学广. 抗冻剂抗冻机制的近红外光谱与分子模拟研究★[J]. 化学学报, 2023, 81(9): 1167-1174. |
| [5] | 侯威, 么艳彩, 张礼知. 电化学还原去除水中含氧酸根离子研究进展★[J]. 化学学报, 2023, 81(8): 979-989. |
| [6] | 杨地, 史潇凡, 张冀杰, 卜显和. 光热材料在海水淡化领域的近期研究进展与展望★[J]. 化学学报, 2023, 81(8): 1052-1063. |
| [7] | 杨宇洁, 巩宇锈, 顾天航, 张伟贤. 冷冻电子显微镜技术进展及环境研究应用★[J]. 化学学报, 2023, 81(8): 990-1001. |
| [8] | 刘祯钰, 甘利华. 乙炔热解为富勒烯的分子动力学模拟研究[J]. 化学学报, 2023, 81(5): 502-510. |
| [9] | 张慧颖, 于淑艳, 李从举. 高分子聚合物基碳纳米膜的电催化降解污水性能及机理[J]. 化学学报, 2023, 81(4): 420-430. |
| [10] | 王文涛, 赖欣婷, 闫士全, 朱雷, 姚玉元, 丁黎明. 双功能气凝胶吸附-降解协同处理染料废水[J]. 化学学报, 2023, 81(3): 222-230. |
| [11] | 张恒杰, 柳坤锐, 陈显春, 顾志鹏, 李乙文. 光响应智能生物粘附材料的设计与应用★[J]. 化学学报, 2023, 81(12): 1739-1753. |
| [12] | 杨镇鸿, 干晓娟, 王书哲, 段君元, 翟天佑, 刘友文. 金属性Ni3N纳米粒子的制备与乙二醇电氧化性能★[J]. 化学学报, 2023, 81(11): 1471-1477. |
| [13] | 韩逸之, 蓝建慧, 刘学, 石伟群. 基于机器学习势函数的熔盐体系分子动力学研究进展[J]. 化学学报, 2023, 81(11): 1663-1672. |
| [14] | 赵珂, 程夏宇, 马雪雪, 耿明慧. 含哌嗪基团锌离子探针的双光子吸收增强机理[J]. 化学学报, 2023, 81(10): 1371-1378. |
| [15] | 王庆鑫, 崔勇, 李蕴琪, 卢善富, 相艳. Fe-N-C阴极催化层离聚物可控热解对膜电极性能与稳定性的影响研究★[J]. 化学学报, 2023, 81(10): 1350-1356. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||