化学学报 ›› 2023, Vol. 81 ›› Issue (9): 1113-1119.DOI: 10.6023/A23050211 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究通讯
投稿日期:2023-05-06
发布日期:2023-06-27
作者简介:基金资助:
Zhengchu Zhang, Wei Xiong( ), Hua Lu(
), Hua Lu( )
)
Received:2023-05-06
Published:2023-06-27
Contact:
*E-mail: About author:Supported by:文章分享

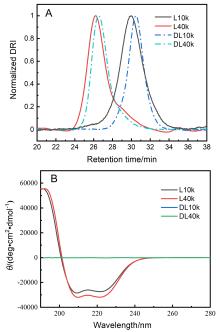
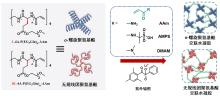
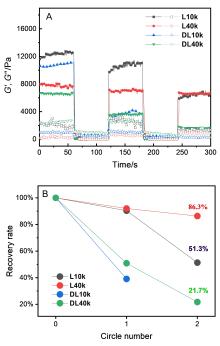
聚氨基酸作为一种主链结构和天然蛋白质相同的合成高分子, 能够形成多种二级结构, 为调节材料的宏观性能提供了新的维度. 为系统研究二级结构对聚氨基酸材料的影响, 本工作分别设计了α-螺旋和无规线团结构的聚氨基酸交联剂, 并构建了两类以不同二级结构聚氨基酸交联的水凝胶. 通过比较两类水凝胶的流变和拉伸性能, 研究了聚氨基酸的α-螺旋结构对水凝胶力学性质的影响. 结果表明, 与无规线团聚氨基酸交联的水凝胶相比, 螺旋聚氨基酸交联的水凝胶表现出更大的刚性、更高的韧性和快速恢复的特征, 展现出α-螺旋作为分子弹簧的特性与作为高力学性能水凝胶增强元件的潜力.
张正初, 熊炜, 吕华. α-螺旋聚氨基酸交联的水凝胶的制备和材料特性★[J]. 化学学报, 2023, 81(9): 1113-1119.
Zhengchu Zhang, Wei Xiong, Hua Lu. Preparation and Material Properties ofα-Helical Polypeptides Crosslinked Hydrogel★[J]. Acta Chimica Sinica, 2023, 81(9): 1113-1119.


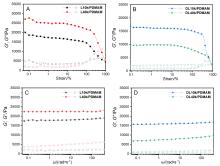

| 凝胶名称 | 储能模量G'/kPa | 耗散模量G"/kPa | 临界应变γ |
|---|---|---|---|
| L10k/PDMAM | 17.5 | 1.7 | 500% |
| L40k/PDMAM | 22.4 | 3.3 | 450% |
| DL10k/PDMAM | 16.0 | 2.3 | >1000% |
| DL40k/PDMAM | 7.8 | 0.8 | >1000% |
| 凝胶名称 | 储能模量G'/kPa | 耗散模量G"/kPa | 临界应变γ |
|---|---|---|---|
| L10k/PDMAM | 17.5 | 1.7 | 500% |
| L40k/PDMAM | 22.4 | 3.3 | 450% |
| DL10k/PDMAM | 16.0 | 2.3 | >1000% |
| DL40k/PDMAM | 7.8 | 0.8 | >1000% |

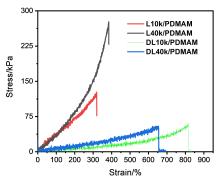

| 凝胶名称 | 弹性模量E/kPa | 断裂能/(kJ•m-3) | 断裂伸长率 |
|---|---|---|---|
| L10k/PDMAM | 34 | 22.4 | 320% |
| L40k/PDMAM | 32 | 43.6 | 350% |
| DL10k/PDMAM | 5.5 | 17.0 | 810% |
| DL40k/PDMAM | 7.1 | 17.2 | 650% |
| 凝胶名称 | 弹性模量E/kPa | 断裂能/(kJ•m-3) | 断裂伸长率 |
|---|---|---|---|
| L10k/PDMAM | 34 | 22.4 | 320% |
| L40k/PDMAM | 32 | 43.6 | 350% |
| DL10k/PDMAM | 5.5 | 17.0 | 810% |
| DL40k/PDMAM | 7.1 | 17.2 | 650% |
| [1] |
Ahmed, E. M. J. Adv. Res. 2015, 6, 105.
doi: 10.1016/j.jare.2013.07.006 pmid: 25750745 |
| [2] |
Zhang, Y. S.; Khademhosseini, A. Science 2017, 356, 3637.
|
| [3] |
Yu, H. C.; Zheng, S. Y.; Fang, L.; Ying, Z.; Du, M.; Wang, J.; Ren, K.; Wu, Z. L.; Zheng, Q. Adv. Mater. 2020, 32, 2005171.
doi: 10.1002/adma.v32.49 |
| [4] |
Han, Y.; Liu, C.; Xu, H.; Cao, Y. Chin. J. Chem. 2022, 40, 1578.
doi: 10.1002/cjoc.v40.13 |
| [5] |
Boehnke, N.; Cam, C.; Bat, E.; Segura, T.; Maynard, H. D. Biomacromolecules 2015, 16, 2101.
doi: 10.1021/acs.biomac.5b00519 |
| [6] |
Lee, K. Y.; Mooney, D. J. Chem. Rev. 2001, 101, 1869.
doi: 10.1021/cr000108x pmid: 11710233 |
| [7] |
Qiu, Y.; Park, K. Adv. Drug Deliv. Rev. 2001, 53, 321.
doi: 10.1016/S0169-409X(01)00203-4 |
| [8] |
Li, J.; Mooney, D. J. Nat. Rev. Mater. 2016, 1, 16071.
doi: 10.1038/natrevmats.2016.71 |
| [9] |
Wang, S. H.; Sun, L. N.; Cao, H.; Zhong, Y. M.; Shao, Z. Z. Acta Chim. Sinica 2021, 79, 1023. (in Chinese)
doi: 10.6023/A21050203 |
|
(王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中, 化学学报, 2021, 79, 1023.)
doi: 10.6023/A21050203 |
|
| [10] |
Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Chem. Rev. 2021, 121, 4309.
doi: 10.1021/acs.chemrev.0c01088 |
| [11] |
Taylor, D.; O’Mara, N.; Ryan, E.; Takaza, M.; Simms, C. Biomed. Mater. 2012, 6, 139.
|
| [12] |
Li, L.; Zhang, K.; Wang, T.; Wang, P.; Xue, B.; Cao, Y.; Zhu, L.; Jiang, Q. Mater. Des. 2020, 189, 108492.
doi: 10.1016/j.matdes.2020.108492 |
| [13] |
Wei, H.; Zhang, B.; Lei, M.; Lu, Z.; Liu, J.; Guo, B.; Yu, Y. ACS Nano 2022, 16, 4734.
doi: 10.1021/acsnano.1c11589 |
| [14] |
Cai, Y.; Shi, J.; Liu, F.; Li, H.; Man, X.; Guan, S. Chin. J. Chem. 2021, 39, 3085.
doi: 10.1002/cjoc.v39.11 |
| [15] |
Yang, Y. Y.; Wang, X.; Wu, D. C. Acta Chim. Sinica 2021, 79, 1. (in Chinese)
doi: 10.6023/A20080370 |
|
(杨艳宇, 王星, 吴德成, 化学学报, 2021, 79, 1.)
doi: 10.6023/A20080370 |
|
| [16] |
Fantner, G. E.; Oroudjev, E.; Schitter, G.; Golde, L. S.; Thurner, P.; Finch, M. M.; Turner, P.; Gutsmann, T.; Morse, D. E.; Hansma, H.; Hansma, P. K. Biophys. J. 2006, 90, 1411.
pmid: 16326907 |
| [17] |
Root, D. D.; Yadavalli, V. K.; Forbes, J. G.; Wang, K. Biophys. J. 2006, 90, 2852.
doi: 10.1529/biophysj.105.071597 |
| [18] |
Xu, Z.-H.; Li, X. Adv. Funct. Mater. 2011, 21, 3883.
doi: 10.1002/adfm.v21.20 |
| [19] |
Tian, Z.-Y.; Zhang, Z.; Wang, S.; Lu, H. Nat. Commun. 2021, 12, 1.
doi: 10.1038/s41467-020-20314-w |
| [20] |
Oelker, A. M.; Morey, S. M.; Griffith, L. G.; Hammond, P. T. Soft Matter 2012, 8, 10887.
doi: 10.1039/c2sm26487k |
| [21] |
Chen, C.; Lan, J.; Li, Y.; Liang, D.; Ni, X.; Liu, Q. Chem. Mater. 2019, 32, 1153.
doi: 10.1021/acs.chemmater.9b04160 |
| [22] |
Liu, R.; Wang, H.; Lu, W.; Cui, L.; Wang, S.; Wang, Y.; Chen, Q.; Guan, Y.; Zhang, Y. Chem. Eng. J. 2021, 415, 128839.
doi: 10.1016/j.cej.2021.128839 |
| [23] |
Liu, P.; Zhang, Y.; Guan, Y.; Zhang, Y. Adv. Mater. 2023, 2210021.
|
| [24] |
Liu, R.; Cui, L.; Wang, H.; Chen, Q.; Guan, Y.; Zhang, Y. ACS Appl. Mater. Interfaces 2021, 13, 42052.
doi: 10.1021/acsami.1c12687 |
| [25] |
Hou, Y.; Zhou, Y.; Wang, H.; Sun, J.; Wang, R.; Sheng, K.; Yuan, J.; Hu, Y.; Chao, Y.; Liu, Z.; Lu, H. ACS Cent. Sci. 2019, 5, 229.
doi: 10.1021/acscentsci.8b00548 |
| [26] |
Malkoch, M.; Vestberg, R.; Gupta, N.; Mespouille, L.; Dubois, P.; Mason, A. F.; Hedrick, J. L.; Liao, Q.; Frank, C. W.; Kingsbury, K.; Hawker, C. J. Chem. Commun. 2006, 26, 2774.
|
| [27] |
Sakai, T.; Akagi, Y.; Kondo, S.; Chung, U. Soft Matter 2014, 10, 6658.
doi: 10.1039/C4SM00709C |
| [28] |
Nele, V.; Wojciechowski, J. P.; Armstrong, J. P.; Stevens, M. M. Adv. Funct. Mater. 2020, 30, 2002759.
doi: 10.1002/adfm.v30.42 |
| [29] |
Song, G.; Zhao, Z.; Peng, X.; He, C.; Weiss, R. A.; Wang, H. Macromolecules 2016, 49, 8265.
doi: 10.1021/acs.macromol.6b01448 |
| [30] |
Wilcox, K. G.; Dingle, M. E.; Saha, A.; Hore, M. J. A.; Morozova, S. Soft Matter 2022, 18, 6550.
doi: 10.1039/D2SM00921H |
| [31] |
Morton, L. D.; Hillsley, A.; Austin, M. J.; Rosales, A. M. J. Mater. Chem. B 2020, 8, 6925.
doi: 10.1039/d0tb00683a pmid: 32436556 |
| [32] |
Zhang, C.; Yuan, J.; Lu, J.; Hou, Y.; Xiong, W.; Lu, H. Biomaterials 2018, 178, 728.
doi: 10.1016/j.biomaterials.2018.01.052 |
| [1] | 史雨晴, 储名珠, 韩波, 马豪杰, 李然, 侯雪艳, 张玉琦, 王记江. 智能二维光子晶体水凝胶精准检测Hg2+[J]. 化学学报, 2024, 82(1): 9-15. |
| [2] | 张恒杰, 柳坤锐, 陈显春, 顾志鹏, 李乙文. 光响应智能生物粘附材料的设计与应用★[J]. 化学学报, 2023, 81(12): 1739-1753. |
| [3] | 王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中. 双载药丝蛋白水凝胶的构筑及其释药性能研究[J]. 化学学报, 2021, 79(8): 1023-1029. |
| [4] | 杨艳宇, 王星, 吴德成. 基于壳聚糖物理网络的高强韧双网络水凝胶的构建、调控与应用[J]. 化学学报, 2021, 79(1): 1-9. |
| [5] | 耿慧敏, 崔基炜, 郝京诚. 仿贻贝水凝胶在组织愈合中的应用研究[J]. 化学学报, 2020, 78(2): 105-113. |
| [6] | 张依, 陈湧, 李晶晶, 梁璐, 刘育. 白光发射超分子水凝胶的构筑和发光性能研究[J]. 化学学报, 2018, 76(8): 622-626. |
| [7] | 邵昱, 李闯, 周旭, 陈平, 杨忠强, 李志波, 刘冬生. 基于G-四联体的聚多肽-DNA水凝胶响应性研究[J]. 化学学报, 2015, 73(8): 815-818. |
| [8] | 任锴, 何金林, 张明祖, 吴一弦, 倪沛红. 酸敏感型嵌段共聚物mPEG-acetal-PIB的合成、表征及用于构筑水凝胶敷料[J]. 化学学报, 2015, 73(10): 1038-1046. |
| [9] | 刘水莲, 周洋, 陈福花, 朱寿进, 宿烽, 李速明. 新型羧甲基壳聚糖水凝胶流变性能,药物释放及细胞相容性研究[J]. 化学学报, 2015, 73(1): 47-52. |
| [10] | 刘艳伟, 曹洪玉, 唐乾, 郑学仿. 大分子拥挤条件下光诱导细胞色素C还原的光谱研究[J]. 化学学报, 2014, 72(2): 246-252. |
| [11] | 金莎, 潘元佳, 汪长春. 回流沉淀聚合:单分散聚合物纳米水凝胶微球制备新技术[J]. 化学学报, 2013, 71(11): 1500-1504. |
| [12] | 张亚玲, 杨斌, 许亮鑫, 张小勇, 陶磊, 危岩. 基于动态化学的自愈性水凝胶及其在生物医用材料中的应用研究展望[J]. 化学学报, 2013, 71(04): 485-492. |
| [13] | 张小军, 刘尚钟, 吴学民, 李姝静. 基于环糊精二聚体与紫精聚合物的包结作用制备超分子水凝胶[J]. 化学学报, 2012, 70(19): 2066-2072. |
| [14] | 王振, 郭东升, 张捷, 刘育. 基于杯芳烃和紫精的电刺激响应二元水凝胶[J]. 化学学报, 2012, 70(16): 1709-1715. |
| [15] | 李姝静, 张小军, 梁海燕, 王心蕊. 基于环糊精二聚体与金刚烷修饰的温敏性聚合物的主客体识别构筑超分子水凝胶[J]. 化学学报, 2012, 70(08): 1013-1020. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||