化学学报 ›› 2025, Vol. 83 ›› Issue (8): 844-852.DOI: 10.6023/A25030072 上一篇 下一篇
研究论文
陆继承a, 苟蕾a, 刘小九a, 樊小勇a, 李东林a,b,*( )
)
投稿日期:2025-03-12
发布日期:2025-06-18
通讯作者:
李东林
基金资助:
Jicheng Lua, Lei Goua, Xiaojiu Liua, Xiaoyong Fana, Donglin Lia,b,*( )
)
Received:2025-03-12
Published:2025-06-18
Contact:
Donglin Li
Supported by:文章分享
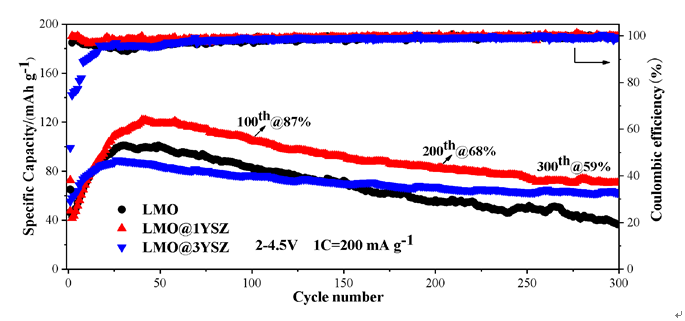
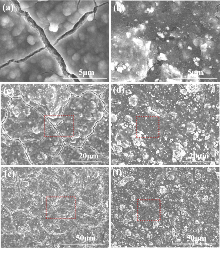
具有正交结构的层状LiMnO2 (LMO)是一种资源充足和廉价的锂离子电池锰基正极材料, 但存在着充放电循环稳定性差等难题. 针对层状LMO正极材料在充放电过程中循环稳定性很差的问题, 本工作采用湿化学法用钇稳定的二氧化锆(Zr0.92Y0.08O2, 或YSZ)铁弹材料对 LMO粉体进行包覆改性, 以提高其充放电循环性能. X射线衍射(XRD)与透射电子显微镜(TEM)分析显示, YSZ为四方结构, 其尺寸分布在3~15 nm, 以薄层或颗粒包覆在LMO颗粒上, 形成了LMO@YSZ复合材料. 在200 mA•g−1电流密度下循环300次, LMO@YSZ复合材料容量保持率为59%, 与纯LiMnO2的36%相比, 明显抑制了其充放电比容量的衰减. 扫描电子显微镜(SEM)分析显示, LMO@YSZ复合材料电极片中的裂纹数量和开裂程度得到明显抑制. 而高分辨电子显微镜显示, 纳米尺寸YSZ存在着四方相的90°铁弹畴. 从实验结果得到推论, LMO@YSZ复合材料充放电循环性能的提高归因于裂纹减少或抑制, 这源于充放电过程中LiMnO2正极材料与Zr0.92Y0.08O2铁弹材料发生电化学-铁弹效应, YSZ的铁弹相变耗散了正极活性材料中的应力能, 有效抑制电极中的裂纹产生, 进而提高了LiMnO2电极的充放电循环性能.
陆继承, 苟蕾, 刘小九, 樊小勇, 李东林. 铁弹Zr0.92Y0.08O2对层状LiMnO2正极材料充放电循环行为的影响[J]. 化学学报, 2025, 83(8): 844-852.
Jicheng Lu, Lei Gou, Xiaojiu Liu, Xiaoyong Fan, Donglin Li. Effect of Ferroelastic Zr0.92Y0.08O2 on the Charge-Discharge Cycling Performances of the Layered LiMnO2 Cathode Materials[J]. Acta Chimica Sinica, 2025, 83(8): 844-852.






| 电极名称 | 初始充电比容量/ (mAh•g−1) | 初始放电比容量/ (mAh•g−1) | 库伦效率 |
|---|---|---|---|
| LMO | 350.60 | 138.17 | 39.40% |
| LMO@1YSZ | 323.28 | 157.60 | 48.75% |
| LMO@3YSZ | 321.62 | 113.13 | 35.17% |
| 电极名称 | 初始充电比容量/ (mAh•g−1) | 初始放电比容量/ (mAh•g−1) | 库伦效率 |
|---|---|---|---|
| LMO | 350.60 | 138.17 | 39.40% |
| LMO@1YSZ | 323.28 | 157.60 | 48.75% |
| LMO@3YSZ | 321.62 | 113.13 | 35.17% |





| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
doi: 10.1149/2.0491813jes |
| [19] |
|
| [20] |
|
| [21] |
|
|
(杨清, 李俊宝, 杨丽, 田鹤, 电子显微学报, 2022, 41, 8.)
|
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
|
(赵蒙, 贾秋阳, 刘瀚文, 雷宇雄, 潘伟, 稀有金属材料与工程, 2013, 42, 473.)
|
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
|
(杨志琴, 杨飞, 刘荣, 南京师范大学学报(工程技术版), 2010, 10, 49.)
|
|
| [31] |
|
|
(粟智, 叶世海, 王永龙, 化学学报, 2009, 67, 2413.)
|
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
|
(刘立虎, 陈述林, 刘凡, 向全军, 冯雄汉, 邱国红, 无机化学学报, 2015, 31, 703.)
|
|
| [38] |
|
| [39] |
|
|
(范广新, 曾跃武, 陈荣升, 吕光烈, 无机化学学报, 2008, 24, 944.)
|
|
| [40] |
|
| [41] |
|
| [42] |
|
|
(苟蕾, 杨哲祺, 余金花, 樊小勇, 李东林, 李辉, 高等学校化学学报, 2024, 45, 20240259.)
doi: 10.7503/cjcu20240259 |
| [1] | 韦正兵, 鲍梦凡, 徐世彪, 程怡, 陈诗洁, 林娜, 冒爱琴. 氟掺杂诱导岩盐型高熵氧化物本征缺陷调控及其储锂性能优化[J]. 化学学报, 2025, 83(8): 868-877. |
| [2] | 薛兰, 刘爽, 章硕, 伽龙. Ni2+掺杂改性对LiMn0.75Fe0.25PO4正极材料电化学性能的影响[J]. 化学学报, 2025, 83(8): 853-860. |
| [3] | 墨云鹤, 曹卫刚, 赵乐泉, 唐振强, 郑珑, 蔡宗英. 基于机器学习与第一性原理筛选锂离子电池钒基电极材料[J]. 化学学报, 2025, 83(5): 518-526. |
| [4] | 郑坤贵, 刘君珂, 胡轶旸, 尹祖伟, 周尧, 李君涛, 孙世刚. 用于石墨负极的高性能聚丙烯酸锂基复合粘结剂的制备及性能研究[J]. 化学学报, 2024, 82(8): 833-842. |
| [5] | 王筑城, 刘磊, 朱梦媛, 孙悦, 赵晴, 丁玉寅, 陆继鑫, 王存国, 李奇, 贺爱华, 叶付臣. 1,5-二氨基蒽醌(AAQ)复合材料用作锂离子电池新型正极材料的性能研究[J]. 化学学报, 2024, 82(6): 589-595. |
| [6] | 顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151. |
| [7] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [8] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [9] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [10] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [11] | 何家伟, 焦柳, 程雪怡, 陈光海, 吴强, 王喜章, 杨立军, 胡征. 金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究[J]. 化学学报, 2022, 80(7): 896-902. |
| [12] | 陈守潇, 刘君珂, 郑伟琛, 魏国祯, 周尧, 李君涛. 电/离子导体双包覆的LiNi0.8Co0.1Mn0.1O2锂离子电池阴极材料及其电化学性能[J]. 化学学报, 2022, 80(4): 485-493. |
| [13] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [14] | 薛晓兰, 张洋, 石美瑜, 李天琳, 黄天龙, 戚继球, 委福祥, 隋艳伟, 金钟. 有机电极材料在非水系金属镁二次电池中的研究进展[J]. 化学学报, 2022, 80(12): 1618-1628. |
| [15] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||