化学学报 ›› 2026, Vol. 84 ›› Issue (1): 30-42.DOI: 10.6023/A25060224 上一篇 下一篇
研究论文
姚志豪a,b, 张巍a,b,*( ), 周昭仪a,b, 李丹聪a,b, 张凯凯a,b, 刘涛a,b, 胡文凯a,b, 程守安a,b, 胡铭轩a,b, 刘昱佳a,b
), 周昭仪a,b, 李丹聪a,b, 张凯凯a,b, 刘涛a,b, 胡文凯a,b, 程守安a,b, 胡铭轩a,b, 刘昱佳a,b
投稿日期:2025-06-17
发布日期:2025-08-18
基金资助:
Zhihao Yaoa,b, Wei Zhanga,b,*( ), Zhaoyi Zhoua,b, Dancong Lia,b, Kaikai Zhanga,b, Tao Liua,b, Wenkai Hua,b, Shouan Chenga,b, Mingxuan Hua,b, Yujia Liua,b
), Zhaoyi Zhoua,b, Dancong Lia,b, Kaikai Zhanga,b, Tao Liua,b, Wenkai Hua,b, Shouan Chenga,b, Mingxuan Hua,b, Yujia Liua,b
Received:2025-06-17
Published:2025-08-18
Contact:
* E-mail: weizhang@csust.edu.cn
Supported by:文章分享
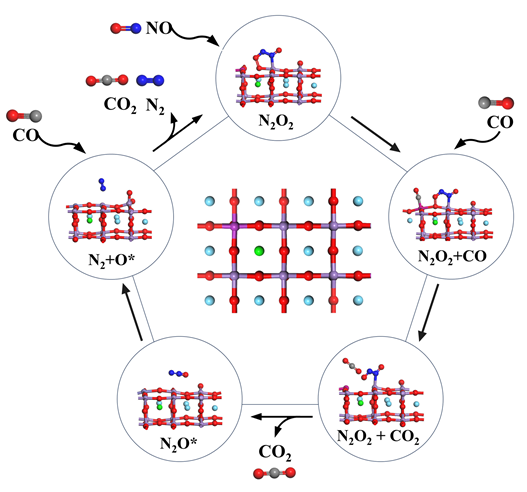
本研究基于密度泛函理论系统探究了Sr/Fe掺杂对LaMnO3钙钛矿的CO选择性催化还原反应机理及抗毒性能机制. 结果表明, Fe单掺杂、Sr-Fe共掺杂有效提高了CO和NO分子在催化剂表面的吸附能力, 而Sr单掺杂会抑制NO在催化剂表面的吸附. Fe掺杂使表面Fe位点成为CO氧化活性中心, 而Mn位点则主导NO的吸附. 在LaSrMnFeO3表面中, CO优先与晶格氧反应生成CO2并形成氧空位, 随后NO吸附形成N2O2*中间体填补氧空位, 活化能垒为0.69 eV, 形成N2O*中间体, 气相的N2O吸附在催化剂表面Lewis酸位点, 解离产生N2*和表面吸附氧, 最终, CO与表面吸附氧克服了0.21 eV活化能垒发生氧化反应, 推动N2O*→N2*+O*反应平衡向右移动, 从而显著抑制N2O*副产物的积累. 上述反应表明, LaSrMnFeO3催化剂表面的CO选择性催化还原(CO-SCR)核心反应机制遵循Mars-van Krevelen机制, 由双分子机制向Mars-van Krevelen机制转变, 其中关键速控步骤N2O*→N2*+O*的反应能垒较未掺杂LaMnO3的双分子反应机制的1.64 eV显著降低至1.14 eV. 此外, 还研究了H2O和SO2在LaMnO3掺杂体系催化剂表面上的吸附性能, 发现Fe单掺杂能够有效提高催化剂的抗硫性能, 但会加剧H2O分子的不可逆吸附导致催化剂中毒失活. 而Sr-Fe共掺杂的协同效应使其同时具备优异抗水和抗硫性能, 为设计高效抗中毒钙钛矿催化剂提供了理论依据.
姚志豪, 张巍, 周昭仪, 李丹聪, 张凯凯, 刘涛, 胡文凯, 程守安, 胡铭轩, 刘昱佳. Sr/Fe协同调控LaMnO3电子结构及CO选择性催化还原反应机理研究[J]. 化学学报, 2026, 84(1): 30-42.
Zhihao Yao, Wei Zhang, Zhaoyi Zhou, Dancong Li, Kaikai Zhang, Tao Liu, Wenkai Hu, Shouan Cheng, Mingxuan Hu, Yujia Liu. Study on Synergistic Modulation of LaMnO3 Electronic Structure and CO Selective Catalytic Reduction Reaction Mechanism via Sr/Fe[J]. Acta Chimica Sinica, 2026, 84(1): 30-42.
| dC-O/nm | dC-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-CO-Mn01 | 0.114 | 0.211 | –0.35 |
| LaSrMnO3-CO-Mn01 | 0.114 | 0.207 | –0.44 |
| LaMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn3 | 0.114 | 0.212 | –0.30 |
| LaMnFeO3-CO-Fe | 0.116 | 0.175 | –0.85 |
| LaSrMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.49 |
| LaSrMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.41 |
| LaSrMnFeO3-CO-Mn3 | 0.114 | 0.208 | –0.31 |
| LaSrMnFeO3-CO-Fe | 0.116 | 0.174 | –0.63 |
| dC-O/nm | dC-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-CO-Mn01 | 0.114 | 0.211 | –0.35 |
| LaSrMnO3-CO-Mn01 | 0.114 | 0.207 | –0.44 |
| LaMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.43 |
| LaMnFeO3-CO-Mn3 | 0.114 | 0.212 | –0.30 |
| LaMnFeO3-CO-Fe | 0.116 | 0.175 | –0.85 |
| LaSrMnFeO3-CO-Mn1 | 0.114 | 0.202 | –0.49 |
| LaSrMnFeO3-CO-Mn2 | 0.114 | 0.202 | –0.41 |
| LaSrMnFeO3-CO-Mn3 | 0.114 | 0.208 | –0.31 |
| LaSrMnFeO3-CO-Fe | 0.116 | 0.174 | –0.63 |
| Q(Sr)/e | Q(Fe)/e | Q(C)/e | Q(O)/e | Q(Mn/Fe)/e | ΔQCO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-CO | — | — | –0.292 | 0.246 | –1.659 | –0.046 |
| LaSrMnO3-CO | –1.659 | — | –0.061 | 0.022 | –1.710 | –0.039 |
| LaMnFeO3-CO | — | –1.364 | –0.120 | 0.238 | –1.544 | 0.118 |
| LaSrMnFeO3-CO | –1.660 | –1.436 | –0.126 | 0.236 | –1.608 | 0.110 |
| Q(Sr)/e | Q(Fe)/e | Q(C)/e | Q(O)/e | Q(Mn/Fe)/e | ΔQCO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-CO | — | — | –0.292 | 0.246 | –1.659 | –0.046 |
| LaSrMnO3-CO | –1.659 | — | –0.061 | 0.022 | –1.710 | –0.039 |
| LaMnFeO3-CO | — | –1.364 | –0.120 | 0.238 | –1.544 | 0.118 |
| LaSrMnFeO3-CO | –1.660 | –1.436 | –0.126 | 0.236 | –1.608 | 0.110 |
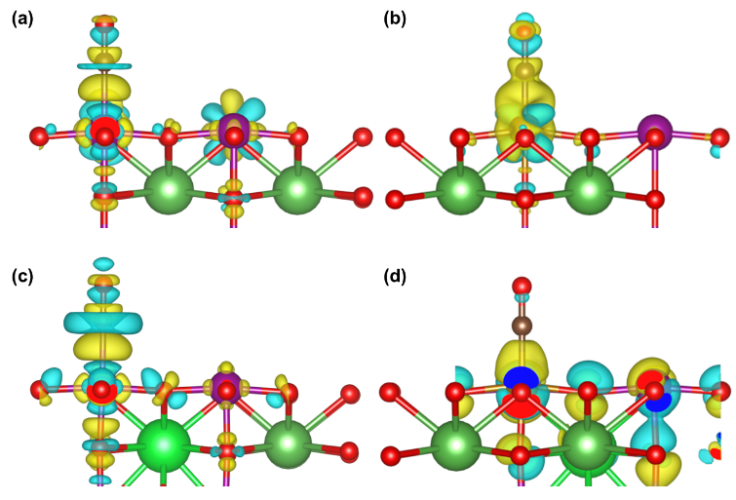
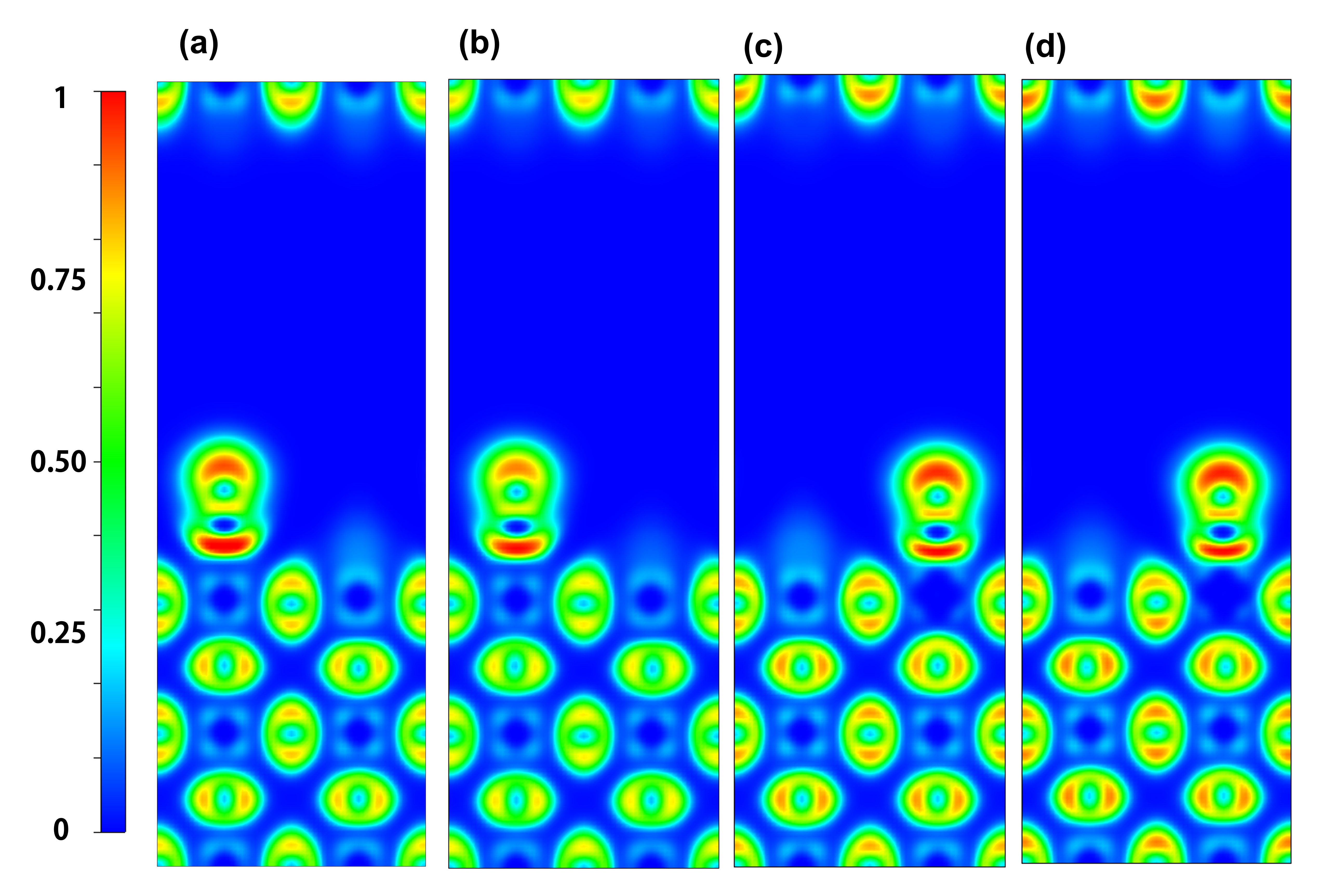

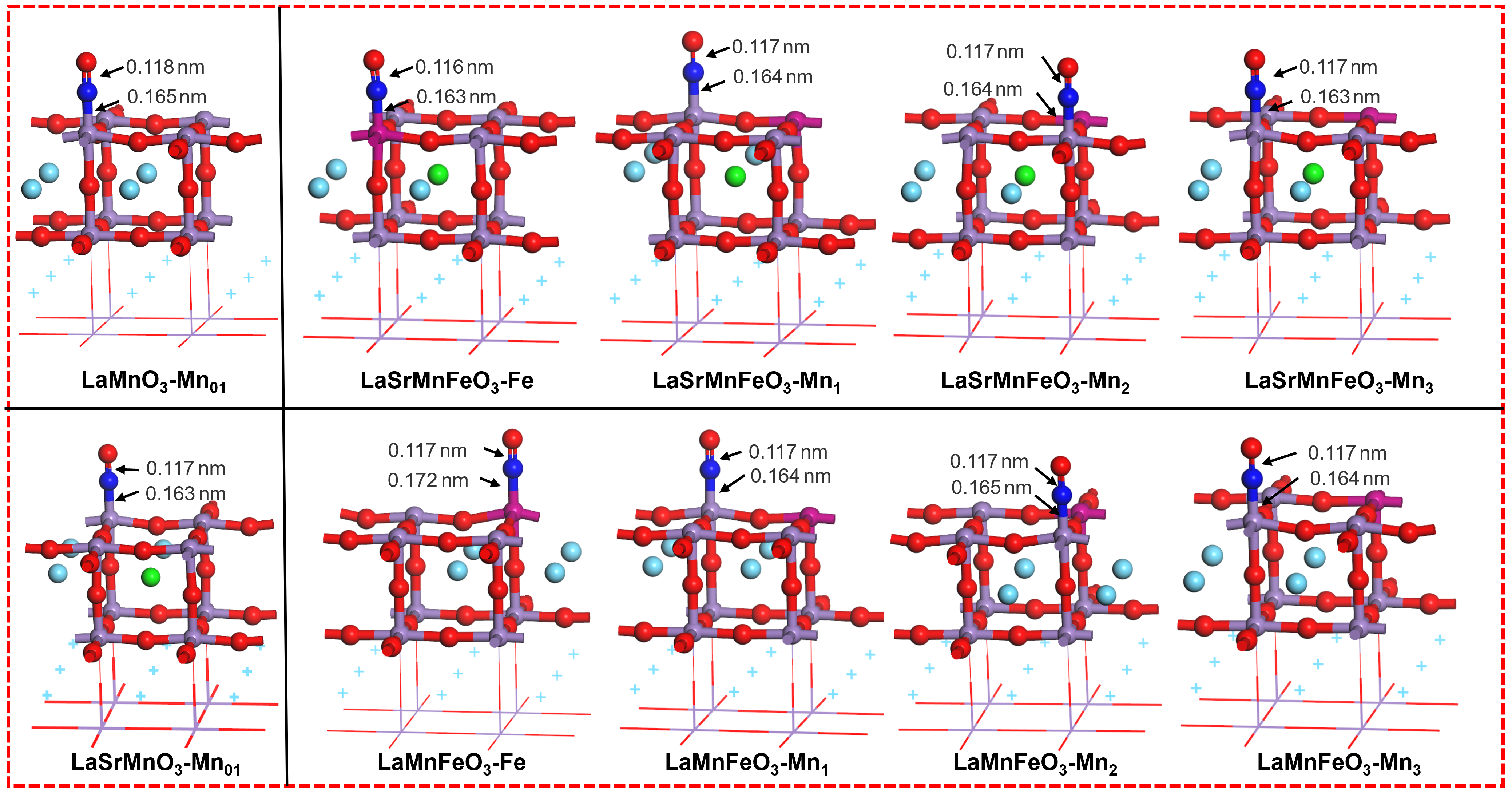
| dN-O/nm | dN-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-NO-Mn01 | 0.118 | 0.165 | –1.88 |
| LaSrMnO3-NO-Mn01 | 0.117 | 0.163 | –1.76 |
| LaMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.93 |
| LaMnFeO3-NO-Mn2 | 0.117 | 0.165 | –1.93 |
| LaMnFeO3-NO-Mn3 | 0.117 | 0.164 | –1.83 |
| LaMnFeO3-NO-Fe | 0.117 | 0.172 | –1.85 |
| LaSrMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.79 |
| LaSrMnFeO3-NO-Mn2 | 0.117 | 0.164 | –1.78 |
| LaSrMnFeO3-NO-Mn3 | 0.117 | 0.163 | –1.74 |
| LaSrMnFeO3-NO-Fe | 0.116 | 0.163 | –1.54 |
| dN-O/nm | dN-metal/nm | Eads/eV | |
|---|---|---|---|
| LaMnO3-NO-Mn01 | 0.118 | 0.165 | –1.88 |
| LaSrMnO3-NO-Mn01 | 0.117 | 0.163 | –1.76 |
| LaMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.93 |
| LaMnFeO3-NO-Mn2 | 0.117 | 0.165 | –1.93 |
| LaMnFeO3-NO-Mn3 | 0.117 | 0.164 | –1.83 |
| LaMnFeO3-NO-Fe | 0.117 | 0.172 | –1.85 |
| LaSrMnFeO3-NO-Mn1 | 0.117 | 0.164 | –1.79 |
| LaSrMnFeO3-NO-Mn2 | 0.117 | 0.164 | –1.78 |
| LaSrMnFeO3-NO-Mn3 | 0.117 | 0.163 | –1.74 |
| LaSrMnFeO3-NO-Fe | 0.116 | 0.163 | –1.54 |
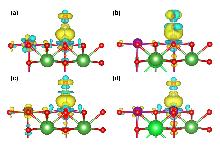
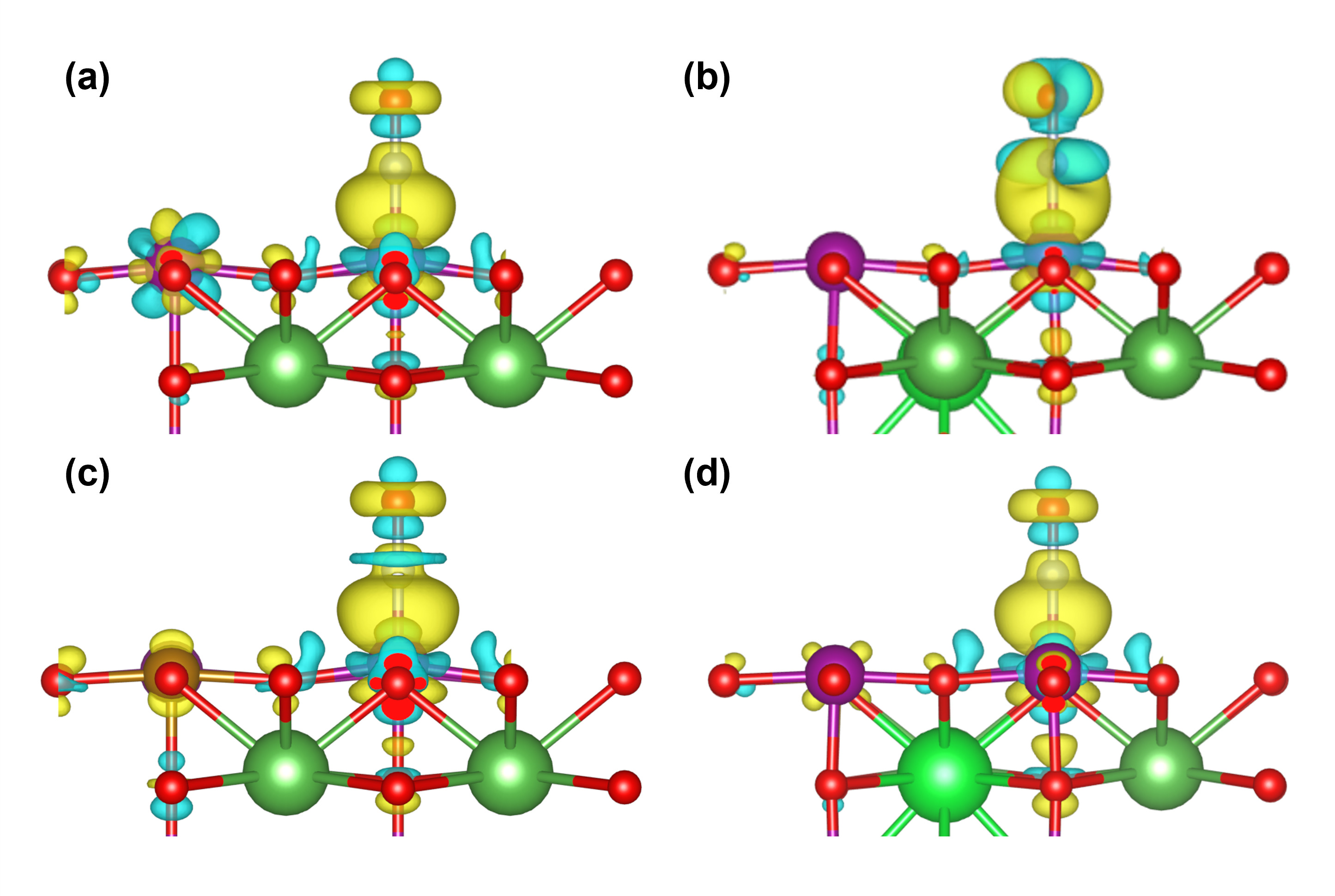
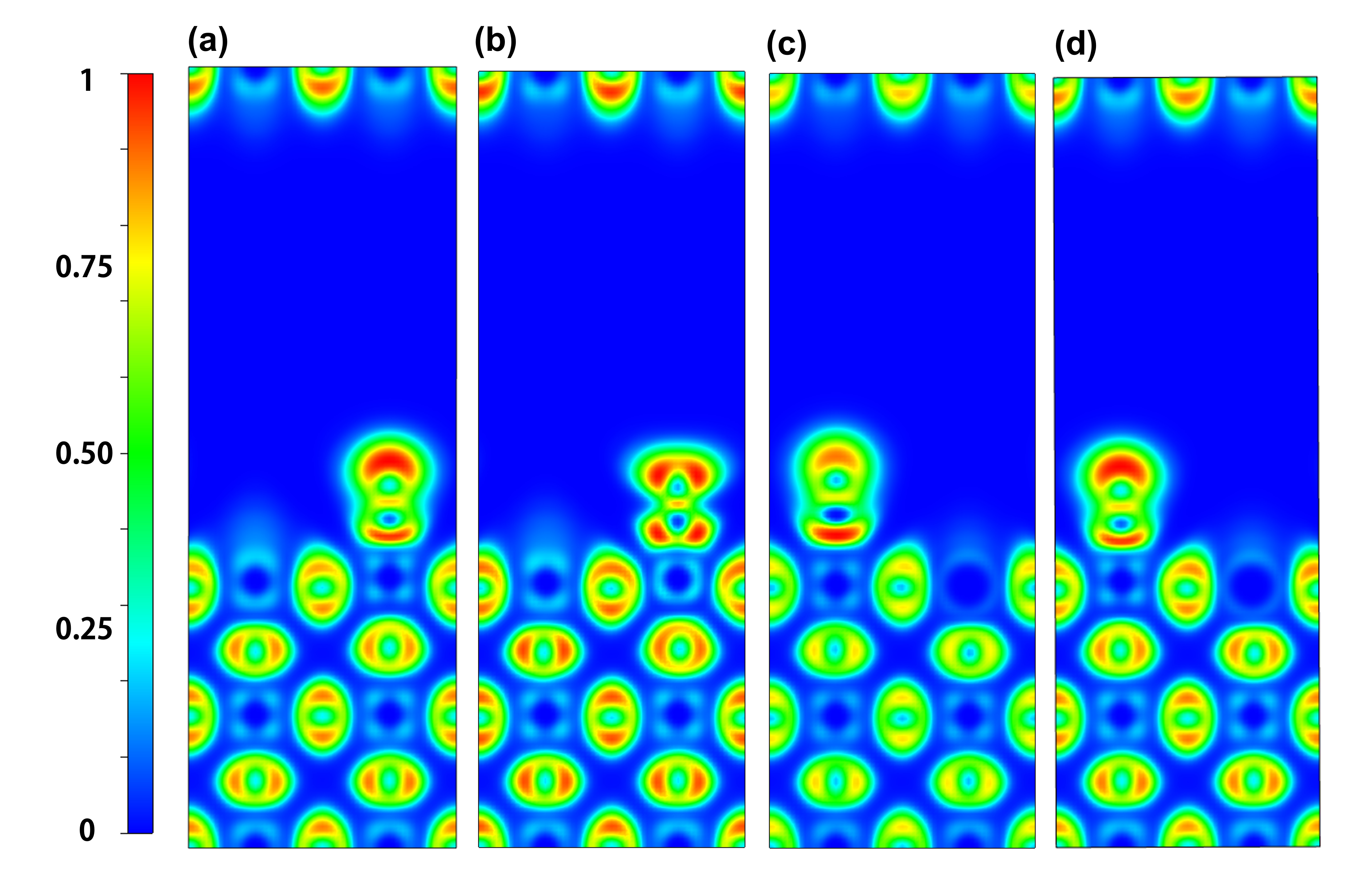
| Q(Sr)/e | Q(Fe)/e | Q(N)/e | Q(O)/e | Q(Mn)/e | ΔQNO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-NO | — | — | 0.112 | 0.135 | –1.636 | 0.245 |
| LaSrMnO3-NO | –1.664 | — | 0.106 | 0.126 | –1.634 | 0.226 |
| LaMnFeO3-NO | — | –1.601 | 0.110 | 0.146 | –1.641 | 0.256 |
| LaSrMnFeO3-NO | –1.658 | –1.591 | 0.148 | 0.101 | –1.698 | 0.249 |
| Q(Sr)/e | Q(Fe)/e | Q(N)/e | Q(O)/e | Q(Mn)/e | ΔQNO/e | |
|---|---|---|---|---|---|---|
| LaMnO3-NO | — | — | 0.112 | 0.135 | –1.636 | 0.245 |
| LaSrMnO3-NO | –1.664 | — | 0.106 | 0.126 | –1.634 | 0.226 |
| LaMnFeO3-NO | — | –1.601 | 0.110 | 0.146 | –1.641 | 0.256 |
| LaSrMnFeO3-NO | –1.658 | –1.591 | 0.148 | 0.101 | –1.698 | 0.249 |






| [1] |
doi: 10.6023/A19040129 |
|
(武卓敏, 石勇, 李春艳, 牛丹阳, 楚奇, 熊巍, 李新勇, 化学学报, 2019, 77, 758.)
doi: 10.6023/A19040129 |
|
| [2] |
doi: 10.6023/A20100478 |
|
(张雅祺, 楚奇, 石勇, 高金索, 熊巍, 黄磊, 丁越, 化学学报, 2021, 79, 361.)
doi: 10.6023/A20100478 |
|
| [3] |
doi: 10.1016/j.jcat.2024.115925 |
| [4] |
doi: 10.1016/j.jece.2024.113593 |
| [5] |
doi: 10.1016/j.joei.2022.07.006 |
| [6] |
doi: 10.1016/j.jece.2025.115568 |
| [7] |
doi: 10.1016/j.fuproc.2021.106798 |
| [8] |
|
|
(赵明新, 洪嘉奇, 王莹, 齐瑞杰, 刘晓刚, 赵田田, 王虹, 低碳化学与化工, 2023, 48, 85.)
|
|
| [9] |
doi: 10.3390/en16227609 |
| [10] |
doi: 10.1016/j.apcatb.2024.124846 |
| [11] |
doi: 10.1016/j.apcata.2023.119231 |
| [12] |
doi: 10.1016/j.cej.2019.04.178 |
| [13] |
doi: 10.1016/j.jece.2021.106612 |
| [14] |
doi: 10.1016/j.molcata.2006.05.008 |
| [15] |
doi: 10.1016/j.jre.2020.12.017 |
| [16] |
doi: 10.1016/j.seppur.2024.127272 |
| [17] |
doi: 10.1016/j.apsusc.2019.145158 |
| [18] |
doi: 10.1007/s10562-016-1860-0 |
| [19] |
doi: 10.1021/acs.iecr.9b01088 |
| [20] |
doi: 10.1039/C9TA09764C |
| [21] |
doi: 10.1016/j.fuel.2024.133891 |
| [22] |
doi: 10.1103/PhysRevB.88.174426 |
| [23] |
|
|
(董抒华, 田贵山, 冯柳, 中国有色金属学报, 2008, 1353.)
|
|
| [24] |
doi: 10.1038/s41929-021-00656-4 |
| [25] |
doi: 10.1016/j.apsusc.2021.151234 |
| [26] |
doi: 10.1016/j.apsusc.2025.163057 |
| [27] |
doi: 10.1016/j.jhazmat.2020.123576 |
| [28] |
doi: 10.1016/0021-9797(87)90248-7 |
| [29] |
doi: 10.1016/S0920-5861(00)00300-X |
| [30] |
|
|
(徐鲁华, 吴晓东, 陈尊, 翁端, 中国稀土学报, 2002, (4), 378.)
|
|
| [31] |
doi: 10.1016/j.cej.2023.141814 |
| [32] |
doi: 10.1016/j.envres.2023.118037 |
| [33] |
doi: 10.1016/j.apcatb.2015.09.002 |
| [34] |
|
| [35] |
doi: 10.1016/j.apcatb.2025.125066 |
| [36] |
doi: 10.1016/S1872-2067(20)63666-X |
| [37] |
doi: 10.1021/acs.iecr.3c01971 |
| [38] |
doi: 10.1016/j.fuel.2019.02.113 |
| [39] |
|
| [1] | 楼一淳, 何承溧, 王霖锐, 崔晓莉. 常温常压下N2、CO2和H2O体系TiO2机械化学合成尿素的实验与理论研究[J]. 化学学报, 2026, 84(1): 73-85. |
| [2] | 韦正兵, 鲍梦凡, 徐世彪, 程怡, 陈诗洁, 林娜, 冒爱琴. 氟掺杂诱导岩盐型高熵氧化物本征缺陷调控及其储锂性能优化[J]. 化学学报, 2025, 83(8): 868-877. |
| [3] | 徐梦鑫, 杨智健, 孙径, 邹文强, 徐忠宁, 郭国聪. Pd/Pr-CeO2催化剂载体氧空位调控CO酯化制碳酸二甲酯选择性[J]. 化学学报, 2025, 83(7): 655-660. |
| [4] | 张娜娜, 李静. 氧空位增强PdNi/HfO2催化剂在乙二醇电催化氧化中的活性[J]. 化学学报, 2025, 83(7): 709-715. |
| [5] | 王鑫, 史燚威, 杨瑞杰, 宋志国, 王敏. 含苯磺酸类配体的单核Cd(II)配合物催化无溶剂“一锅法”Biginelli反应[J]. 化学学报, 2025, 83(7): 674-684. |
| [6] | 卢一林, 董盛杰, 崔方超, 薄婷婷, 毛卓. 希托夫紫磷烯/SnS2范德华异质结作为直接全解水光催化剂的理论构建[J]. 化学学报, 2025, 83(4): 377-389. |
| [7] | 孙伟, 辛国祥, 刘飞, 鞠藤, 程宇通, 宋金玲, 包金小, 布林朝克. 三维石墨烯/富含氧空位Fe2O3复合材料的构建实现超级电容器超高能量密度[J]. 化学学报, 2025, 83(3): 256-265. |
| [8] | 陈铭晖, 张博心, 魏滔, 孙兆雪, 冯亚青, 张宝. 三嗪共价骨架材料的层间位错行为及其光生载流子动力学理论研究[J]. 化学学报, 2025, 83(2): 93-100. |
| [9] | 张乃心, 石伟群, 王聪芝. 二价锕系配合物AnB8的理论研究[J]. 化学学报, 2025, 83(12): 1523-1529. |
| [10] | 马莹, 陈维希, 刘羽辰, 刘子义, 吴涛, 陆安慧, 王东琪. 六方氮化硼氧化模式的密度泛函理论研究[J]. 化学学报, 2025, 83(1): 52-59. |
| [11] | 张帆帆, 蔡元韬, 陶剑波, 常国菊, 郭欣辰, 郝仕油. Zn, C引入量和煅烧温度对ZnO/C/CeO2光催化还原Cu2+效率的影响[J]. 化学学报, 2024, 82(8): 871-878. |
| [12] | 王治业, 肖博怀. 利用平面σ-芳香性增强电子输运能力[J]. 化学学报, 2024, 82(5): 520-526. |
| [13] | 赵雨晴, 梁栋, 贾吉慧, 余荣民, 卢灿忠. 具有双吸电子基团D-A型配体的Ag(I)发光配合物的合成与性能研究[J]. 化学学报, 2024, 82(5): 486-492. |
| [14] | 王国景, 陈永辉, 张秀芹, 张俊笙, 徐俊敏, 王静. 氧空位控制BiVO4晶面异质结的磁性和光电催化性能[J]. 化学学报, 2024, 82(4): 409-415. |
| [15] | 赵玉强, 张霞, 杨芸如, 朱立平, 周莹. 聚集诱导发射光笼分子的设计合成及原位光激活成像研究[J]. 化学学报, 2024, 82(3): 265-273. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||