化学学报 ›› 2025, Vol. 83 ›› Issue (12): 1523-1529.DOI: 10.6023/A25080276 上一篇 下一篇
研究论文
张乃心a,b, 石伟群a,b,c,*( ), 王聪芝b,*(
), 王聪芝b,*( )
)
投稿日期:2025-08-10
发布日期:2025-10-10
基金资助:
Naixin Zhanga,b, Weiqun Shia,b,c,*( ), Congzhi Wangb,*(
), Congzhi Wangb,*( )
)
Received:2025-08-10
Published:2025-10-10
Contact:
* E-mail: shiwq@ihep.ac.cn;wangcongzhi@ihep.ac.cn
Supported by:文章分享

由于硼元素具有缺电子特性, 随尺寸变化硼团簇呈现多种多样的结构, 不同金属的掺杂能够进一步丰富硼团簇的结构和性质. 通过全局最优结构搜索和密度泛函理论(DFT)方法研究了一系列锕系金属掺杂硼团簇AnB8 (An=Ac, Th, Am, Cm). 研究发现, 每个AnB8团簇都具有半夹心和椅状结构两种构型, 除ThB8外, AcB8、AmB8和CmB8的半夹心结构最稳定, 而ThB8的两种结构能量差异较小. 这些硼团簇的半夹心结构均为稳定的MII[${B}_{8}^{2-}$]型二价锕系配合物, 而且${B}_{8}^{2-}$配体具有σ和π双重芳香性. 成键性质分析表明, 所有配合物中An—B之间存在共价相互作用, 而且ThB8的共价相互作用更强. 所有配合物都具有较高的稳定性, 尤其是ThB8. 这些结果表明, ${B}_{8}^{2-}$配体能够稳定二价锕系元素.
张乃心, 石伟群, 王聪芝. 二价锕系配合物AnB8的理论研究[J]. 化学学报, 2025, 83(12): 1523-1529.
Naixin Zhang, Weiqun Shi, Congzhi Wang. Theoretical Studies of Divalent Actinide Complexes AnB8[J]. Acta Chimica Sinica, 2025, 83(12): 1523-1529.
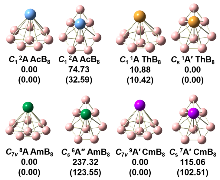

| 硼团簇 | 自旋态 | ⟨S2⟩计算值-理论值 | 自旋密度 ρAn | 键长/nm | |
|---|---|---|---|---|---|
| An—B1 | An—Ba | ||||
| AcB8 | 双重态 | 0.00 | 0.77 | 0.2750 | 0.3017 |
| ThB8 | 单重态 | — | — | 0.2485 | 0.2729 |
| AmB8 | 八重态 | 0.02 | 7.14 | 0.2573 | 0.2882 |
| CmB8 | 九重态 | 0.03 | 8.10 | 0.2588 | 0.2849 |
| 硼团簇 | 自旋态 | ⟨S2⟩计算值-理论值 | 自旋密度 ρAn | 键长/nm | |
|---|---|---|---|---|---|
| An—B1 | An—Ba | ||||
| AcB8 | 双重态 | 0.00 | 0.77 | 0.2750 | 0.3017 |
| ThB8 | 单重态 | — | — | 0.2485 | 0.2729 |
| AmB8 | 八重态 | 0.02 | 7.14 | 0.2573 | 0.2882 |
| CmB8 | 九重态 | 0.03 | 8.10 | 0.2588 | 0.2849 |
| 硼团簇 | 原子电荷/a.u. | WBIs | SOMO-LUMO 能隙/eV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VDD | Hirshfeld | An—B1 | An—Ba | α | β | |||||
| AcB8 | 0.743 | 0.796 | 0.620 | 0.421 | 1.20 | 3.45 | ||||
| ThB8 | 0.746 | 0.723 | 0.611 | 0.516 | 1.49 | — | ||||
| AmB8 | 0.766 | 0.807 | 0.638 | 0.346 | 2.91 | 3.50 | ||||
| CmB8 | 0.607 | 0.636 | 0.631 | 0.425 | 2.16 | 3.81 | ||||
| 硼团簇 | 原子电荷/a.u. | WBIs | SOMO-LUMO 能隙/eV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VDD | Hirshfeld | An—B1 | An—Ba | α | β | |||||
| AcB8 | 0.743 | 0.796 | 0.620 | 0.421 | 1.20 | 3.45 | ||||
| ThB8 | 0.746 | 0.723 | 0.611 | 0.516 | 1.49 | — | ||||
| AmB8 | 0.766 | 0.807 | 0.638 | 0.346 | 2.91 | 3.50 | ||||
| CmB8 | 0.607 | 0.636 | 0.631 | 0.425 | 2.16 | 3.81 | ||||
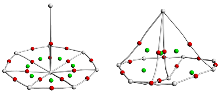
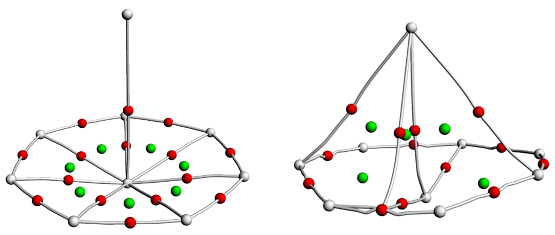
| 硼团簇 | ρ | H | ∇2ρ | ELF | DItotal |
|---|---|---|---|---|---|
| AcB8 | 0.03987 | -0.00481 | 0.07581 | 0.24085 | 2.387 |
| ThB8 | 0.06165 | -0.01692 | 0.06650 | 0.40489 | 3.628 |
| AmB8 | 0.04167 | -0.00736 | 0.11279 | 0.14418 | 1.800 |
| CmB8 | 0.04180 | -0.00726 | 0.11792 | 0.13616 | 2.226 |
| 硼团簇 | ρ | H | ∇2ρ | ELF | DItotal |
|---|---|---|---|---|---|
| AcB8 | 0.03987 | -0.00481 | 0.07581 | 0.24085 | 2.387 |
| ThB8 | 0.06165 | -0.01692 | 0.06650 | 0.40489 | 3.628 |
| AmB8 | 0.04167 | -0.00736 | 0.11279 | 0.14418 | 1.800 |
| CmB8 | 0.04180 | -0.00726 | 0.11792 | 0.13616 | 2.226 |
| 反应方程 | 解离能 |
|---|---|
| AcB8→Ac2++${B}_{8}^{2-}$ | 2055.6 |
| ThB8→Th2++${B}_{8}^{2-}$ | 2384.0 |
| AmB8→Am2++${B}_{8}^{2-}$ | 1988.2 |
| CmB8→Cm2++${B}_{8}^{2-}$ | 2190.7 |
| 反应方程 | 解离能 |
|---|---|
| AcB8→Ac2++${B}_{8}^{2-}$ | 2055.6 |
| ThB8→Th2++${B}_{8}^{2-}$ | 2384.0 |
| AmB8→Am2++${B}_{8}^{2-}$ | 1988.2 |
| CmB8→Cm2++${B}_{8}^{2-}$ | 2190.7 |
| [1] |
doi: 10.1021/ja507235s |
| [2] |
doi: 10.1038/nchem.1999 |
| [3] |
doi: 10.1038/ncomms4113 |
| [4] |
doi: 10.1002/anie.v42:48 |
| [5] |
doi: 10.1002/anie.v46:44 |
| [6] |
doi: 10.1038/nmat1012 |
| [7] |
pmid: 17111395 |
| [8] |
doi: 10.1038/ncomms9654 pmid: 26456760 |
| [9] |
doi: 10.1039/D0NR09214B |
| [10] |
doi: 10.1039/C7NR02399E |
| [11] |
doi: 10.1021/ja802494z pmid: 18479137 |
| [12] |
doi: 10.1002/anie.v50.40 |
| [13] |
doi: 10.1039/D4CP04358H |
| [14] |
doi: 10.1039/C9CS00233B |
| [15] |
doi: 10.1038/s41570-017-0071 |
| [16] |
doi: 10.1039/D1CS00747E |
| [17] |
|
| [18] |
doi: 10.1021/acs.accounts.4c00380 |
| [19] |
doi: 10.1002/anie.201107880 pmid: 22298320 |
| [20] |
doi: 10.1021/jp108668t |
| [21] |
doi: 10.1039/C9CP03611C |
| [22] |
doi: 10.1039/C8CP01376D |
| [23] |
doi: 10.1016/j.chemphys.2019.02.008 |
| [24] |
doi: 10.6023/A22030109 |
|
(李海茹, 张层, 李思殿, 化学学报, 2022, 80, 888.)
doi: 10.6023/A22030109 |
|
| [25] |
doi: 10.1039/D1CC07303F |
| [26] |
doi: 10.1116/6.0001833 |
| [27] |
doi: 10.1063/1.3625959 |
| [28] |
pmid: 15180405 |
| [29] |
doi: 10.1039/D4CP00296B |
| [30] |
doi: 10.1021/acs.jpca.1c05846 |
| [31] |
|
| [32] |
doi: 10.1039/D1CP05058C |
| [33] |
doi: 10.1039/D4CP01646G |
| [34] |
doi: 10.3390/molecules29235815 |
| [35] |
doi: 10.1021/ct2006852 |
| [36] |
doi: 10.1021/jp811503v pmid: 19572689 |
| [37] |
doi: 10.1021/acs.jpca.9b04005 |
| [38] |
doi: 10.1039/c5dt04540a pmid: 26777518 |
| [39] |
doi: 10.1039/c0cp01575j pmid: 20967377 |
| [40] |
|
| [41] |
doi: 10.1002/jcc.v25:2 |
| [42] |
doi: 10.1007/BF00549096 |
| [43] |
|
| [44] |
doi: 10.1063/1.458517 |
| [45] |
doi: 10.1039/b804083d pmid: 18728862 |
| [46] |
doi: 10.1103/PhysRevB.82.094116 |
| [47] |
doi: 10.1016/j.cpc.2012.05.008 |
| [48] |
doi: 10.1063/1.4746757 |
| [49] |
doi: 10.1063/1.4769731 |
| [50] |
doi: 10.1103/physrevb.48.13115 pmid: 10007687 |
| [51] |
doi: 10.1103/physrevb.54.11169 pmid: 9984901 |
| [52] |
doi: 10.1039/C7CC09837E |
| [53] |
doi: 10.1063/1.3682776 |
| [54] |
doi: 10.1021/acs.inorgchem.2c00624 |
| [55] |
|
|
(张乃心, 王聪芝, 赵玉宝, 石伟群, 核化学与放射化学, 2022, 44, 549.)
doi: 10.7538/hhx.2022.YX.2021037 |
|
| [56] |
|
|
(张乃心, 王聪芝, 石伟群, 核化学与放射化学, 2023, 45, 160.)
doi: 10.7538/hhx.2022.YX.2021114 |
|
| [57] |
doi: 10.1021/acs.inorgchem.4c02950 pmid: 39231309 |
| [58] |
doi: 10.1021/acs.inorgchem.4c03446 pmid: 39285662 |
| [59] |
|
| [60] |
doi: 10.1002/jcc.v33.5 |
| [61] |
doi: 10.1063/5.0216272 |
| [1] | 姚志豪, 张巍, 周昭仪, 李丹聪, 张凯凯, 刘涛, 胡文凯, 程守安, 胡铭轩, 刘昱佳. Sr/Fe协同调控LaMnO3电子结构及CO选择性催化还原反应机理研究[J]. 化学学报, 2026, 84(1): 30-42. |
| [2] | 楼一淳, 何承溧, 王霖锐, 崔晓莉. 常温常压下N2、CO2和H2O体系TiO2机械化学合成尿素的实验与理论研究[J]. 化学学报, 2026, 84(1): 73-85. |
| [3] | 王鑫, 史燚威, 杨瑞杰, 宋志国, 王敏. 含苯磺酸类配体的单核Cd(II)配合物催化无溶剂“一锅法”Biginelli反应[J]. 化学学报, 2025, 83(7): 674-684. |
| [4] | 卢一林, 董盛杰, 崔方超, 薄婷婷, 毛卓. 希托夫紫磷烯/SnS2范德华异质结作为直接全解水光催化剂的理论构建[J]. 化学学报, 2025, 83(4): 377-389. |
| [5] | 陈铭晖, 张博心, 魏滔, 孙兆雪, 冯亚青, 张宝. 三嗪共价骨架材料的层间位错行为及其光生载流子动力学理论研究[J]. 化学学报, 2025, 83(2): 93-100. |
| [6] | 马莹, 陈维希, 刘羽辰, 刘子义, 吴涛, 陆安慧, 王东琪. 六方氮化硼氧化模式的密度泛函理论研究[J]. 化学学报, 2025, 83(1): 52-59. |
| [7] | 王治业, 肖博怀. 利用平面σ-芳香性增强电子输运能力[J]. 化学学报, 2024, 82(5): 520-526. |
| [8] | 赵雨晴, 梁栋, 贾吉慧, 余荣民, 卢灿忠. 具有双吸电子基团D-A型配体的Ag(I)发光配合物的合成与性能研究[J]. 化学学报, 2024, 82(5): 486-492. |
| [9] | 赵玉强, 张霞, 杨芸如, 朱立平, 周莹. 聚集诱导发射光笼分子的设计合成及原位光激活成像研究[J]. 化学学报, 2024, 82(3): 265-273. |
| [10] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [11] | 黄伊晨, 聂长明, 王聪芝, 陈树森, 宋艳, 李昊, 石伟群. 羟基和氨基取代偕胺肟用于海水提铀的理论研究[J]. 化学学报, 2024, 82(10): 1050-1057. |
| [12] | 梁雪峰, 荆剑, 冯昕, 赵勇泽, 唐新员, 何燕, 张立胜, 李慧芳. 共价有机框架COF66/COF366的电子结构: 从单体到二维平面聚合物[J]. 化学学报, 2023, 81(7): 717-724. |
| [13] | 杨磊, 葛娇阳, 王访丽, 吴汪洋, 郑宗祥, 曹洪涛, 王洲, 冉雪芹, 解令海. 一种基于芴的大环结构的有效降低内重组能的理论研究[J]. 化学学报, 2023, 81(6): 613-619. |
| [14] | 刘康, 郭燕, 于吉攀, 石伟群. 锕系单分子磁体研究进展[J]. 化学学报, 2023, 81(3): 264-274. |
| [15] | 张少秦, 李美清, 周中军, 曲泽星. 多共振热激活延迟荧光过程的理论研究[J]. 化学学报, 2023, 81(2): 124-130. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||