化学学报 ›› 2011, Vol. 69 ›› Issue (18): 2099-2107. 上一篇 下一篇
研究论文
张智1,2,刘朝*,1,李豪杰1,黄金保1,黄晓露1
Zhang Zhi1,2 Liu Chao*,1 Li Haojie1 Huang Jinbao1 Huang Xiaolu1
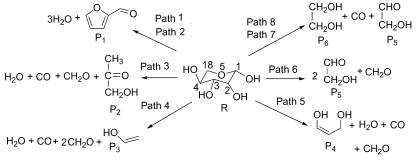
采用Gaussian 03程序中的密度泛函(DFT)理论B3LYP/6-31++G(d, p)方法, 对木聚糖一个循环单元的热解机理进行了理论研究. 设计了八条热解路径, 对每条路径中反应物、中间体、过渡态和产物进行能量梯度全优化, 并对过渡态进行了IRC验证, 在298~1098 K温度范围内计算了每个路径的热力学及动力学参数. 结果表明: Path1, Path2为放热反应, Path3在798 K由吸热反应转变为放热反应, 其余路径为吸热反应|室温到798 K, 2-糠醛和水的转化率最高, 温度高于898 K, Path4成为主反应路径. 通过动力学分析给出了木糖分子不同断键方式下的三个最优路径: Path2的控速步为Step5活化能为236.9 kJ/mol|Path6的控速步为Step7, 活化能为363.9 kJ/mol|Path7或Path8的控速步为Step15, 活化能为336.5 kJ/mol. 分析表明, 无论热力学或是动力学都优先支持Path2生成2-糠醛和水, 其次是Path6生成乙醇醛和甲醛, Path7或Path8生成乙醇醛、乙二醇和一氧化碳.
中图分类号: