化学学报 ›› 2021, Vol. 79 ›› Issue (9): 1164-1172.DOI: 10.6023/A21050236 上一篇 下一篇
研究论文
投稿日期:2021-05-28
发布日期:2021-09-17
通讯作者:
魏思敏
基金资助:
Yinghui Wanga, Simin Weib( ), Jinwei Duana, Kang Wanga
), Jinwei Duana, Kang Wanga
Received:2021-05-28
Published:2021-09-17
Contact:
Simin Wei
Supported by:文章分享

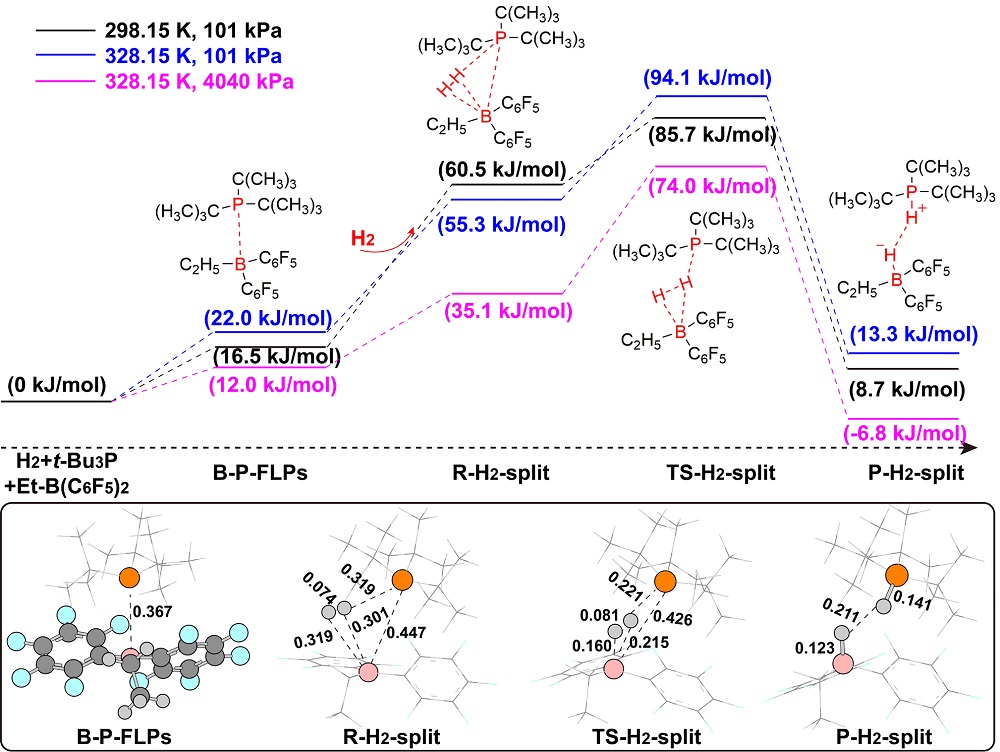
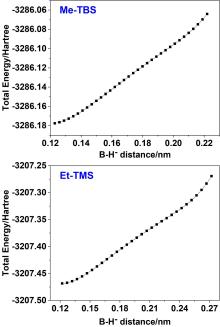
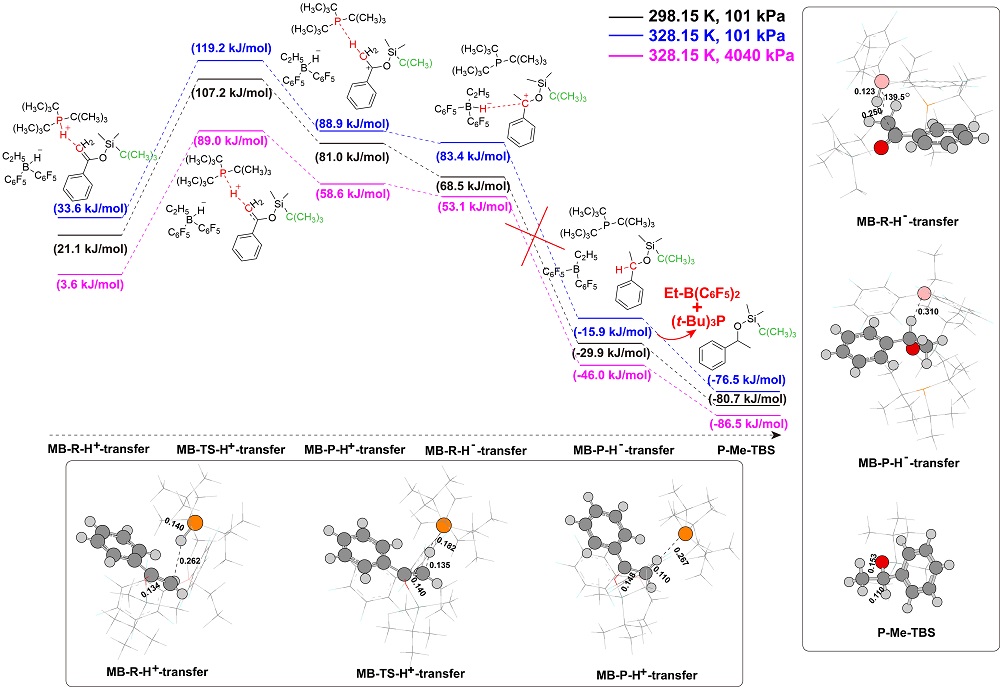
“受阻路易斯酸碱对”(FLPs)催化的烯醇硅醚氢化反应是一类重要的直接合成醇类化合物的方法, 然而目前其反应机理仍不明确. 基于此, 以乙基取代的全氟苯基硼作为路易斯酸(Et-B(C6F5)2), 三叔丁基膦(t-Bu3P)作为路易斯碱, 烯醇硅醚化的苯乙酮(Me-TMS)作为底物建立了模型反应, 并使用密度泛函理论系统研究了其催化氢化反应机理. 结果显示: FLPs催化的烯醇硅醚氢化反应从Et-B(C6F5)2和t-Bu3P形成B-P-FLPs开始, 随后会依次经过H2裂解, H+和H-转移等过程, 其中H+转移为决速步, H-转移无势垒, B-P-FLPs生成及H+转移是吸热反应. 升高温度不利于氢化反应发生, 但是增大压力可促进反应进行. 底物取代基效应会影响H-转移过程, 可能使反应不发生.
王英辉, 魏思敏, 段金伟, 王康. 理论研究“受阻路易斯酸碱对”催化的烯醇硅醚氢化反应机理[J]. 化学学报, 2021, 79(9): 1164-1172.
Yinghui Wang, Simin Wei, Jinwei Duan, Kang Wang. Mechanism of Silyl Enol Ethers Hydrogenation Catalysed by Frustrated Lewis Pairs: A Theoretical Study[J]. Acta Chimica Sinica, 2021, 79(9): 1164-1172.


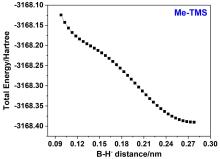
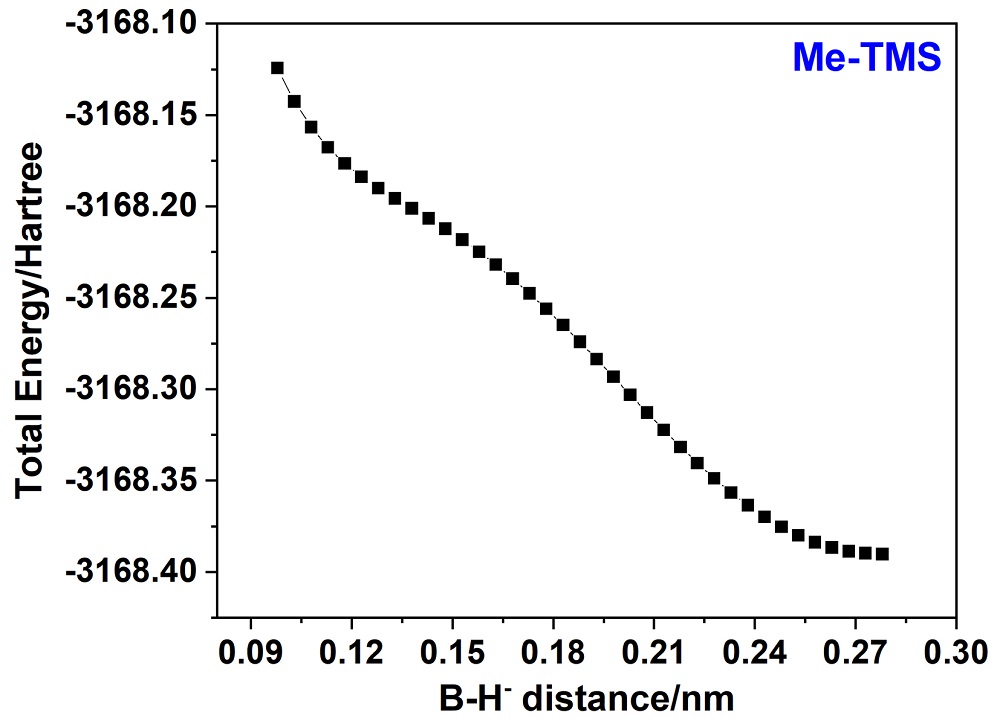


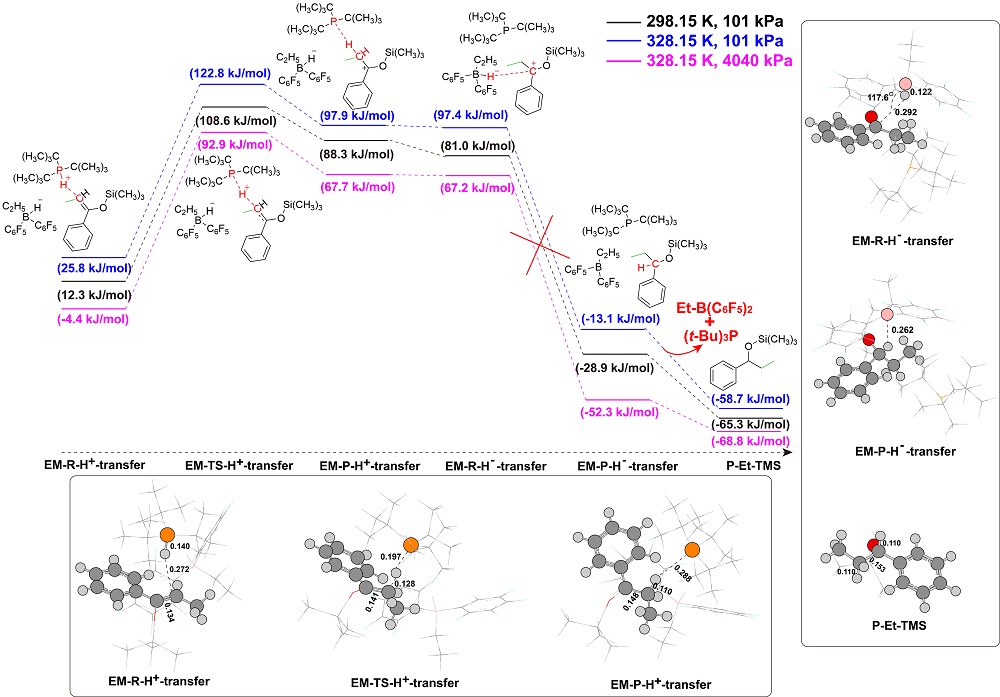


| [1] |
Liu, W. P.; Sahoo, B.; Junge, K.; Beller, M. Acc. Chem. Res. 2018, 51, 1858.
doi: 10.1021/acs.accounts.8b00262 |
| [2] |
Wang, Q. Y.; Santos, S.; Urbina-Blanco, C. A.; Hernandez, W. Y.; Imperor-Clerc, M.; Vovk, E. I.; Marinova, M.; Ersen, O.; Baaziz, W.; Safonova, O. V.; Khodakov, A. Y.; Saeys, M.; Ordomsky, V. V. Appl. Catal. B-Environ. 2021, 290, 120036.
doi: 10.1016/j.apcatb.2021.120036 |
| [3] |
Chen, S. N.; Deng, J.; Ye, C.; Xu, C. C.; Huai, L. Y.; Ling, X.; Li, J.; Li, X. Y. Chem. Eng. J. 2021, 410, 128825.
|
| [4] |
Wang, W. L.; Niu, J. F.; Yang, Z. F. J. Hazard. Mater. 2021, 411, 121912.
|
| [5] |
Ye, R. P.; Lin, L.; Li, Q. H.; Zhou, Z. F.; Wang, T. T.; Russell, C. K.; Adidharma, H.; Xu, Z. H.; Yao, Y. G.; Fan, M. H. Catal. Sci. Technol. 2018, 8, 3428.
doi: 10.1039/C8CY00608C |
| [6] |
Song, J. J.; Huang, Z. F.; Pan, L.; Li, K.; Zhang, X. W.; Wang, L.; Zou, J. J. Appl. Catal. B-Environ. 2018, 227, 386.
doi: 10.1016/j.apcatb.2018.01.052 |
| [7] |
Schreier, M. R.; Pfund, B.; Guo, X. W.; Wenger, O. S. Chem. Sci. 2020, 11, 8582.
doi: 10.1039/d0sc01820a pmid: 34123118 |
| [8] |
Lux, S.; Baldauf-Sommerbauer, G.; Siebenhofer, M. ChemSusChem 2018, 11, 3357.
doi: 10.1002/cssc.v11.19 |
| [9] |
Meemken, F.; Baiker, A. Chem. Rev. 2017, 117, 11522.
doi: 10.1021/acs.chemrev.7b00272 pmid: 28872309 |
| [10] |
Hu, S. B.; Chen, M. W.; Zhai, X. Y.; Zhou, Y. G. Acta Chim. Sinica 2018, 76, 103. (in Chinese)
doi: 10.6023/A17110476 |
|
( 胡书博, 陈木旺, 翟小勇, 周永贵, 化学学报, 2018, 76, 103.)
doi: 10.6023/A17110476 |
|
| [11] |
Liu, X.; Han, Z. B.; Wang, Z.; Ding, K. L. Acta Chim. Sinica 2014, 72, 849. (in Chinese)
doi: 10.6023/A14040314 |
|
( 刘旭, 韩召斌, 王正, 丁奎岭, 化学学报, 2014, 72, 849.)
doi: 10.6023/A14040314 |
|
| [12] |
Liu, Y. B.; Du, H. F. Acta Chim. Sinica 2014, 72, 771. (in Chinese)
doi: 10.6023/A14040344 |
|
( 刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.)
doi: 10.6023/A14040344 |
|
| [13] |
Meemken, F.; Rodriguez-Garcia, L. J. Phys. Chem. Lett. 2018, 9, 996.
doi: 10.1021/acs.jpclett.7b03360 pmid: 29420894 |
| [14] |
Xie, J. H.; Zhou, Q. L. Acta Chim. Sinica 2012, 70, 1427. (in Chinese)
doi: 10.6023/A12060268 |
|
( 谢建华, 周其林, 化学学报, 2012, 70, 1427.)
doi: 10.6023/A12060268 |
|
| [15] |
Zhang, Q.; Liu, A.; Yu, H. Z.; Fu, Y. Acta Chim. Sinica 2018, 76, 113. (in Chinese)
doi: 10.6023/A17070328 |
|
( 张琪, 刘奥, 于海珠, 傅尧, 化学学报, 2018, 76, 113.)
doi: 10.6023/A17070328 |
|
| [16] |
Schauermann, S. J. Phys. Chem. Lett. 2018, 9, 5555.
doi: 10.1021/acs.jpclett.8b01782 pmid: 30204444 |
| [17] |
Bai, Y. P.; Cui, C. M. Acta Chim. Sinica 2020, 78, 763. (in Chinese)
doi: 10.6023/A20050163 |
|
( 白云平, 崔春明, 化学学报, 2020, 78, 763.)
doi: 10.6023/A20050163 |
|
| [18] |
Welch, G. C.; Juan, R. R. S.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
doi: 10.1126/science.1134230 |
| [19] |
Stephan, D. W. Acc. Chem. Res. 2015, 48, 306.
doi: 10.1021/ar500375j |
| [20] |
Mömming, C. M.; Frömel, S.; Kehr, G.; Fröhlich, R.; Grimme, S.; Erker, G. J. Am. Chem. Soc. 2009, 131, 12280.
doi: 10.1021/ja903511s |
| [21] |
Mahdi, T.; Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
doi: 10.1021/ja508829x |
| [22] |
Mahdi, T.; Heiden, Z. M.; Grimme, S.; Stephan, D. W. J. Am. Chem. Soc. 2012, 134, 4088.
doi: 10.1021/ja300228a |
| [23] |
Zhang, Z.; Du, H. Angew. Chem. Int. Ed. 2015, 54, 623.
|
| [24] |
Zhang, Z. H.; Du, H. F. Org. Lett. 2015, 17, 6266.
doi: 10.1021/acs.orglett.5b03307 |
| [25] |
Wei, S. M.; Feng, X. Q.; Du, H. F. Org. Biomol. Chem. 2016, 14, 8026.
doi: 10.1039/C6OB01556E |
| [26] |
Wei, S. M.; Du, H. F. J. Am. Chem. Soc. 2014, 136, 12261.
doi: 10.1021/ja507536n |
| [27] |
Liu, Y. B.; Du, H. F. J. Am. Chem. Soc. 2013, 135, 6810.
doi: 10.1021/ja4025808 |
| [28] |
Liu, Y. B.; Du, H. F. J. Am. Chem. Soc. 2013, 135, 12968.
doi: 10.1021/ja406761j |
| [29] |
Lu, Z. P.; Cheng, Z. H.; Chen, Z. X.; Weng, L. H.; Li, Z. H.; Wang, H. D. Angew. Chem.-Int. Ed. 2011, 50, 12227.
doi: 10.1002/anie.v50.51 |
| [30] |
Liu, Q.; Yang, L.; Yao, C.; Geng, J.; Wu, Y.; Hu, X. Org. Lett. 2021, 23, 3685.
doi: 10.1021/acs.orglett.1c01073 |
| [31] |
Rouf, A. M.; Huang, Y.; Dong, S.; Zhu, J. Inorg. Chem. 2021, 60, 5598.
doi: 10.1021/acs.inorgchem.0c03520 |
| [32] |
Wang, H. L.; Zhang, W. N.; Lu, L.; Liu, D. P.; Liu, D. D.; Li, T. Z.; Yan, S. C.; Zhao, S. Q.; Zou, Z. G. Appl. Catal. B-Environ. 2021, 283, 119639.
doi: 10.1016/j.apcatb.2020.119639 |
| [33] |
Szynkiewicz, N.; Chojnacki, J.; Grubba, R. Inorg. Chem. 2020, 59, 6332.
doi: 10.1021/acs.inorgchem.0c00435 pmid: 32286811 |
| [34] |
Adenot, A.; von Wolff, N.; Lefevre, G.; Berthet, J. C.; Thuery, P.; Cantat, T. Chem.-Eur. J. 2019, 25, 8118.
doi: 10.1002/chem.v25.34 |
| [35] |
Kehr, G.; Erker, G. Chem. Rec. 2017, 17, 803.
doi: 10.1002/tcr.v17.8 |
| [36] |
Wang, H. D.; Frohlich, R.; Kehr, G.; Erker, G. Chem. Commun. 2008, 5966.
|
| [37] |
Greb, L.; Ona-Burgos, P.; Kubas, A.; Falk, F. C.; Breher, F.; Fink, K.; Paradies, J. Dalton Trans. 2012, 41, 9056.
doi: 10.1039/c2dt30374d |
| [38] |
Ren, X. Y.; Du, H. F. J. Am. Chem. Soc. 2016, 138, 810.
doi: 10.1021/jacs.5b13104 |
| [39] |
Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215.
doi: 10.1007/s00214-007-0310-x |
| [40] |
Wang, Y. H.; Jie, J. L.; Zhao, H. M.; Bai, Y.; Qin, P. X.; Song, D. Acta Chim. Sinica 2018, 76, 475. (in Chinese)
doi: 10.6023/A17120557 |
|
( 王英辉, 节家龙, 赵红梅, 白羽, 秦佩萱, 宋迪, 化学学报, 2018, 76, 475.)
doi: 10.6023/A17120557 |
|
| [41] |
Wei, S.; Zhang, Z.; Liu, S.; Wang, Y. New J. Chem. 2021, 45, 11202.
doi: 10.1039/D1NJ01653A |
| [42] |
Huang, F.; Jiang, J. L.; Wen, M. W.; Wang, Z. X. J. Theor. Comput. Chem. 2014, 13, 1350074.
doi: 10.1142/S0219633613500740 |
| [43] |
Wang, Y. H.; Wei, S. M.; Wang, K.; Xu, R. R.; Zhao, H. M. Acta Chim. Sinica 2020, 78, 271. (in Chinese)
doi: 10.6023/A19120435 |
|
( 王英辉, 魏思敏, 王康, 徐蓉蓉, 赵红梅, 化学学报, 2020, 78, 271.)
doi: 10.6023/A19120435 |
|
| [44] |
Zhao, J. Y.; Wang, G. Q.; Li, S. H. Dalton Trans. 2015, 44, 9200.
doi: 10.1039/C5DT00978B |
| [45] |
Rokob, T. A.; Hamza, A.; Stirling, A.; Pápai, I. J. Am. Chem. Soc. 2009, 131, 2029.
doi: 10.1021/ja809125r |
| [46] |
Antinolo, A.; Carrillo-Hermosilla, F.; Fernandez-Galan, R.; Martinez-Ferrer, J.; Alonso-Moreno, C.; Bravo, I.; Moreno-Blazquez, S.; Salgado, M.; Villasenor, E.; Albaladejo, J. Dalton Trans. 2016, 45, 10717.
doi: 10.1039/C6DT01237J |
| [47] |
Zhao, L.; Li, H.; Lu, G.; Huang, F.; Zhang, C.; Wang, Z.-X. Dalton Trans. 2011, 40, 1929.
doi: 10.1039/c0dt01297a |
| [48] |
Rokob, T. A.; Hamza, A.; Papai, I. J. Am. Chem. Soc. 2009, 131, 10701.
doi: 10.1021/ja903878z |
| [49] |
Wei, S. M.; Wang, Y. H.; Zhao, H. M. Acta Chim. Sinica 2019, 77, 278. (in Chinese)
doi: 10.6023/A18110461 |
|
( 魏思敏, 王英辉, 赵红梅, 化学学报, 2019, 77, 278.)
doi: 10.6023/A18110461 |
|
| [50] |
Cances, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032.
doi: 10.1063/1.474659 |
| [51] |
Das, S.; Pati, S. K. Chem.-Eur. J. 2017, 23, 1078.
doi: 10.1002/chem.201602774 |
| [52] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Ha-segawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 16, Revision A. 03, Gaussian, Inc., Wallingford, CT, 2016.
|
| [1] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [2] | 梁雪峰, 荆剑, 冯昕, 赵勇泽, 唐新员, 何燕, 张立胜, 李慧芳. 共价有机框架COF66/COF366的电子结构: 从单体到二维平面聚合物[J]. 化学学报, 2023, 81(7): 717-724. |
| [3] | 杨磊, 葛娇阳, 王访丽, 吴汪洋, 郑宗祥, 曹洪涛, 王洲, 冉雪芹, 解令海. 一种基于芴的大环结构的有效降低内重组能的理论研究[J]. 化学学报, 2023, 81(6): 613-619. |
| [4] | 张少秦, 李美清, 周中军, 曲泽星. 多共振热激活延迟荧光过程的理论研究[J]. 化学学报, 2023, 81(2): 124-130. |
| [5] | 王娟, 肖华敏, 谢丁, 郭元茹, 潘清江. 铜掺杂与氮化碳复合氧化锌材料结构和二氧化氮气体传感性质的密度泛函理论计算[J]. 化学学报, 2023, 81(11): 1493-1499. |
| [6] | 刘金晶, 杨娜, 李莉, 魏子栋. 铂活性位空间结构调控氧还原机理的理论研究★[J]. 化学学报, 2023, 81(11): 1478-1485. |
| [7] | 栾雪菲, 王聪芝, 夏良树, 石伟群. 铀酰与羧酸和肟基类配体相互作用的理论研究[J]. 化学学报, 2022, 80(6): 708-713. |
| [8] | 王珞聪, 李哲伟, 岳彩巍, 张培焕, 雷鸣, 蒲敏. 电场下偶氮苯衍生物分子顺反异构化反应机理的理论研究[J]. 化学学报, 2022, 80(6): 781-787. |
| [9] | 熊昆, 陈伽瑶, 杨娜, 蒋尚坤, 李莉, 魏子栋. 理论探究水溶液条件对TMNxCy催化氮还原性能的增强机制[J]. 化学学报, 2021, 79(9): 1138-1145. |
| [10] | 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良. Cu催化偶联反应合成烷基芳基醚的DFT机理研究[J]. 化学学报, 2021, 79(7): 948-952. |
| [11] | 王岩, 田英齐, 金钟, 索兵兵. 基于GPU的Hartree-Fock与密度泛函算法及程序[J]. 化学学报, 2021, 79(5): 653-657. |
| [12] | 张丹琪, 邵英博, 郑汉良, 周碧莹, 薛小松. 双齿氮配体螯合五价碘试剂介导的苯酚氧化去芳构化机理的理论研究[J]. 化学学报, 2021, 79(11): 1394-1400. |
| [13] | 鲁效庆, 曹守福, 魏晓飞, 李邵仁, 魏淑贤. S掺杂Fe-NC单原子催化剂氧还原机理研究[J]. 化学学报, 2020, 78(9): 1001-1006. |
| [14] | 于沫涵, 程媛媛, 刘亚军. 萤火虫生物发光中加氧反应机理的理论研究[J]. 化学学报, 2020, 78(9): 989-993. |
| [15] | 杜重阳, 陈耀峰. 二乙基锌促进CO2的硅氢化反应以及CO2为C1合成子的有机胺甲酰化或脲化反应[J]. 化学学报, 2020, 78(9): 938-944. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||