化学学报 ›› 2022, Vol. 80 ›› Issue (1): 44-48.DOI: 10.6023/A21100465 上一篇 下一篇
研究论文
投稿日期:2021-10-19
发布日期:2021-11-18
通讯作者:
金艳梅
Yanmei Jina( ), Ye Mengb, Yuan Lia, Jianhua Shia, Lei Denga
), Ye Mengb, Yuan Lia, Jianhua Shia, Lei Denga
Received:2021-10-19
Published:2021-11-18
Contact:
Yanmei Jin
文章分享

本工作以对称二环己基取代六元瓜环(CyH2Q[6])为主体分子, 3-吡啶甲酰肼(NH)为客体分子, 利用核磁共振(1H NMR)、等温滴定量热(ITC)、基质辅助激光解吸电离飞行时间质谱(MALDI-TOF)研究客体分子与瓜环在水溶液中形成的物质的量比为1∶1的稳定配合物; 用X-射线单晶衍射可以观察到客体分子通过离子-偶极和氢键与瓜环端口羰基氧相互作用, 以及基于瓜环外壁正电性与无机阴离子之间形成的配合物, 从而形成多维多层次超分子框架的自组装体.
金艳梅, 蒙叶, 李远, 史建华, 邓雷. 对称二环己基取代六元瓜环与3-吡啶甲酰肼的超分子自组装[J]. 化学学报, 2022, 80(1): 44-48.
Yanmei Jin, Ye Meng, Yuan Li, Jianhua Shi, Lei Deng. Supramolecular Self-assembly of Symmetric Dicyclohexanocucurbit[6]uril and Nicotinic Hydrazide[J]. Acta Chimica Sinica, 2022, 80(1): 44-48.
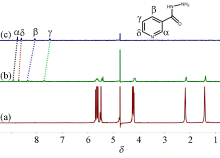
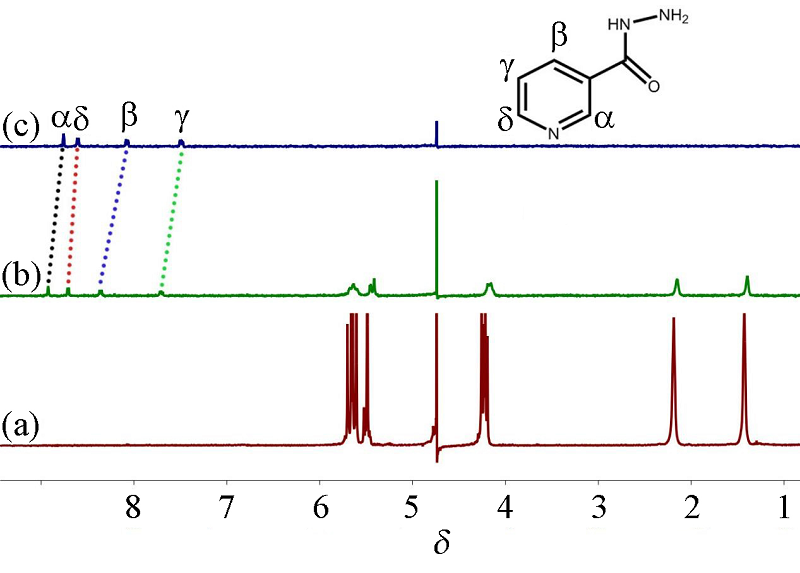
| Experiment | NH@CyH2Q[6] |
|---|---|
| Model | Independent |
| Ka/(L•mol-1) | (1.019±0.118)×103 |
| ΔH/(kJ•mol-1) | –48.21±0.35 |
| n | 0.954±0.013 |
| TΔS/(kJ•mol-1) | –31.04±0.52 |
| Experiment | NH@CyH2Q[6] |
|---|---|
| Model | Independent |
| Ka/(L•mol-1) | (1.019±0.118)×103 |
| ΔH/(kJ•mol-1) | –48.21±0.35 |
| n | 0.954±0.013 |
| TΔS/(kJ•mol-1) | –31.04±0.52 |
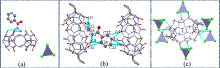
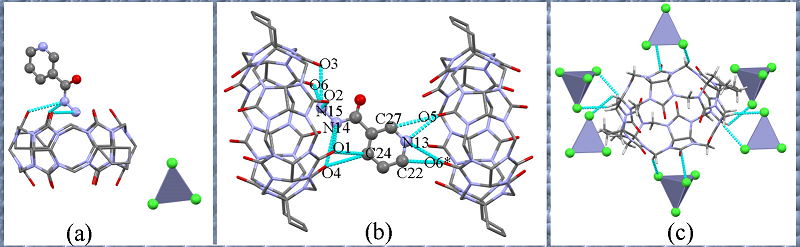
| NH@CyH2Q[6] | |||
|---|---|---|---|
| Bond | Length/nm | Bond | Length/nm |
| N15―O3 | 0.2841 | N13―O5 | 0.2680 |
| N15―O6 | 0.2925 | N13―O6 | 0.3009 |
| N15―O2 | 0.2927 | C22―O6 | 0.2953 |
| N14―O1 | 0.2990 | C10―Cl1 | 0.3318 |
| N14―O4 | 0.3048 | H13A―Cl6 | 0.2739 |
| C24―O1 | 0.3095 | H5―Cl3 | 0.2950 |
| C24―O4 | 0.3035 | H1A―Cl3 | 0.2782 |
| C27―O5 | 0.3162 | H1B―Cl2 | 0.2924 |
| NH@CyH2Q[6] | |||
|---|---|---|---|
| Bond | Length/nm | Bond | Length/nm |
| N15―O3 | 0.2841 | N13―O5 | 0.2680 |
| N15―O6 | 0.2925 | N13―O6 | 0.3009 |
| N15―O2 | 0.2927 | C22―O6 | 0.2953 |
| N14―O1 | 0.2990 | C10―Cl1 | 0.3318 |
| N14―O4 | 0.3048 | H13A―Cl6 | 0.2739 |
| C24―O1 | 0.3095 | H5―Cl3 | 0.2950 |
| C24―O4 | 0.3035 | H1A―Cl3 | 0.2782 |
| C27―O5 | 0.3162 | H1B―Cl2 | 0.2924 |


| NH@CYH2Q[6] | |||
|---|---|---|---|
| Empirical formula | C56H66Zn2Cl8N30O14 | Formula weight | 1797.72 |
| Crystal system | Triclinic | Space group | P-1 |
| a/nm | 1.2304(3) | b/nm | 1.3677(3) |
| c/nm | 1.3896(4) | α/(°) | 71.692(9) |
| β/(°) | 76.013(7) | γ/(°) | 67.789(7) |
| V/nm3 | 2.0352(9) | Z | 1 |
| Dcalcd/(g•cm-3)] | 1.467 | T/K | 298 |
| μ/mm-1 | 0.927 | Parameters | 525 |
| Rint | 0.0994 | R[I>2σ(I)]a | 0.1458 |
| wR[I>2σ(I)]b | 0.3933 | R (all data) | 0.2284 |
| wR (all data) | 0.4437 | GOF on F2 | 1.352 |
| NH@CYH2Q[6] | |||
|---|---|---|---|
| Empirical formula | C56H66Zn2Cl8N30O14 | Formula weight | 1797.72 |
| Crystal system | Triclinic | Space group | P-1 |
| a/nm | 1.2304(3) | b/nm | 1.3677(3) |
| c/nm | 1.3896(4) | α/(°) | 71.692(9) |
| β/(°) | 76.013(7) | γ/(°) | 67.789(7) |
| V/nm3 | 2.0352(9) | Z | 1 |
| Dcalcd/(g•cm-3)] | 1.467 | T/K | 298 |
| μ/mm-1 | 0.927 | Parameters | 525 |
| Rint | 0.0994 | R[I>2σ(I)]a | 0.1458 |
| wR[I>2σ(I)]b | 0.3933 | R (all data) | 0.2284 |
| wR (all data) | 0.4437 | GOF on F2 | 1.352 |
| [1] |
Dusselier, M.; Davis, M. E. Chem. Rev. 2018, 118, 5265.
doi: 10.1021/acs.chemrev.7b00738 |
| [2] |
Helal, A.; Yamani, Z. H.; Cordova, K. E.; Yaghi, O. M. Natl. Sci. Rev. 2017, 4, 296.
doi: 10.1093/nsr/nwx013 |
| [3] |
Yaghi, O. M.; Li, G. M.; Li, H. L. Nature 1995, 378, 703.
doi: 10.1038/378703a0 |
| [4] |
Yang, L.; Wu, Y. J.; Wu, X. J.; Cai, W. Q. Acta Chim. Sinica 2021, 79, 520 ; (in Chinese)
doi: 10.6023/A20110526 |
|
( 杨磊, 吴宇静, 吴选军, 蔡卫权, 化学学报 2021, 79, 520.)
|
|
| [5] |
Wang, H.; Zeng, Z. T.; Xu, P.; Li, L. S.; Zeng, G. G.; Xiao, R.; Tang, Z. Y.; Huang, D. L.; Tang, L.; Lai, C.; Jiang, D. N.; Liu, Y.; Yi, H.; Qin, L.; Ye, S. J.; Ren, X. Y.; Tang, W. W. Chem. Soc. Rev. 2019, 48, 488.
doi: 10.1039/C8CS00376A |
| [6] |
Waller, P. J.; Gandara, F.; Yaghi, O. M. Acc. Chem. Res. 2015, 48, 3053.
doi: 10.1021/acs.accounts.5b00369 |
| [7] |
Liu, J. G.; Zhang, M. Y.; Wang, N.; Wang, C. G.; Ma, L. L. Acta Chim. Sinica 2020, 78, 311 ; (in Chinese)
doi: 10.6023/A19120426 |
|
( 刘建国, 张明月, 王楠, 王晨光, 马隆龙, 化学学报 2020, 78, 311.)
|
|
| [8] |
Wen, Y.; Zhang, J.; Xu, Q.; Wu, X. T.; Zhu, Q. L. Coord. Chem. Rev. 2018, 376, 248.
doi: 10.1016/j.ccr.2018.08.012 |
| [9] |
Chedid, G.; Yassin, A. Nanomaterials 2018, 8, 916.
doi: 10.3390/nano8110916 |
| [10] |
Han, S. S.; Mendoza-Cortes, J. L.; Goddard III, W. A. Chem. Soc. Rev. 2009, 38, 1460.
doi: 10.1039/b802430h |
| [11] |
Kim, J. Y.; Oh, H.; Moon, H. R. Adv. Mater. 2019, 31, 1805293.
doi: 10.1002/adma.v31.20 |
| [12] |
Zhang, X. M.; Li, X. Y.; Xiong, W. F.; Li, H. F.; Cao, R. Acta Chim. Sinica 2021, 79, 180 ; (in Chinese)
doi: 10.6023/A20090445 |
|
( 张晓萌, 李希雅, 熊晚枫, 李红芳, 曹荣, 化学学报 2021, 79, 180.)
|
|
| [13] |
Day, A. I.; Blanck, R. J.; Arnold, A. P. Angew. Chem. Int. Ed. 2002, 41, 275.
|
| [14] |
Kim, J.; Jung, I. S.; Kim, S. Y.; Lee, E.; Kang, J. K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. J. Am. Chem. Soc. 2000, 122, 540.
doi: 10.1021/ja993376p |
| [15] |
Isaacs, L.; Park, S. K.; Liu, S. M.; Ko, Y. H.; Selvapalam, N.; Kim, Y.; Kim, H.; Zavalij, P. Y.; Kim, G. H.; Lee, H. S.; Kim, K. J. Am. Chem. Soc. 2005, 127, 18000.
doi: 10.1021/ja056988k |
| [16] |
Ni, X. L.; Xiao, X.; Cong, H.; Liang, L. L.; Chen, K.; Cheng, X. J.; Ji, N. N.; Zhu, Q. J.; Xue, S. F.; Tao, Z. Chem. Soc. Rev. 2013, 42, 9480.
doi: 10.1039/c3cs60261c |
| [17] |
Li, Q.; Sun, J.; Zhou, J.; Hua, B.; Shao, L.; Huang, F. Org. Chem. Front. 2018, 5, 1940.
doi: 10.1039/C8QO00323H |
| [18] |
Li, Q.; Jie, K.; Huang, F. Angew. Chem. Int. Ed. 2020, 59, 5355.
doi: 10.1002/anie.v59.13 |
| [19] |
Jin, Y. M.; Jiang, D. F.; Meng, Y.; Gao, J.; Zheng, J.; Ma, P. H. J. Incl. Phenom. Macro. 2021, 100, 209.
doi: 10.1007/s10847-021-01076-4 |
| [20] |
Meng, Y.; Zhao, W. W.; Zheng, J.; Jiang, D. F.; Gao, J.; Jin, Y. M.; Ma, P. H. RSC Adv. 2021, 11, 3470.
doi: 10.1039/D0RA09074C |
| [21] |
Wang, C.; Cheng, S. Y.; Zhao, W. W.; Yang, X. N.; Zhou, K. Z.; Tian, J. J.; Jiang, D. F.; Ma, P. H. Crystallogr. Rep. 2020, 65, 1156.
doi: 10.1134/S1063774520070275 |
| [22] |
Zhang, Z. R.; Kan, J. L.; Feng, H. M.; Liu, Q. Y.; Tao, Z.; Xiao, X. Chinese J. Org. Chem. 2018, 38, 1972 ; (in Chinese)
doi: 10.6023/cjoc201804008 |
|
( 张智睿, 阚京兰, 冯华明, 刘青云, 陶朱, 肖昕, 有机化学 2018, 38, 1972.)
|
|
| [23] |
Bai, D.; Zhou, Y.; Lu, J. H.; Liu, Q. Y.; Chen, Q.; Tao, Z.; Xiao, X. Chinese J. Org. Chem. 2018, 38, 1477 ; (in Chinese)
doi: 10.6023/cjoc201801020 |
|
( 白东, 周杨, 卢季红, 刘青云, 陈青, 陶朱, 肖昕, 有机化学 2018, 38, 1477.)
|
|
| [24] |
Freeman, W. A.; Mock, W. L.; Shih, N. Y. J. Am. Chem. Soc. 1981, 103, 7367.
doi: 10.1021/ja00414a070 |
| [25] |
Day, A. I.; Arnold, A. P. WO 0068232, 2000.
|
| [26] |
Zhao, J. Z.; Kim, H. J.; Oh, J.; Kim, S. Y.; Lee, J. W.; Sakamoto, S.; Yamaguchi, K.; Kim, K. Angew. Chem. Int. Ed. 2001, 40, 4233.
|
| [27] |
Jin, Y. M.; Meng, Y.; Yang, X. N.; Zhu, C.; Tao, Z.; Liu, J. X.; Ma, P. H. Cryst. Growth Des. 2021, 21, 2977.
doi: 10.1021/acs.cgd.1c00138 |
| [28] |
Zheng, L. M.; Liu, J. X. J. Solid State Chem. 2017, 245, 45.
doi: 10.1016/j.jssc.2016.10.008 |
| [29] |
Sheldrick, G. M. Acta Crystallogr. Sect. A 2008, 64, 112.
doi: 10.1107/S0108767307043930 |
| [30] |
Sheldrick, G. M. SHELXS-97 and SHELXL-97, University of Goettingen, Germany, 1997.
|
| [31] |
Spek, A. L. J. Appl. Crystallogr. 2003, 36, 7.
doi: 10.1107/S0021889802022112 |
| [1] | 陶鹏, 郑小康, 王国良, 盛星浩, 姜贺, 李文桃, 靳继彪, 王瑞鸿, 苗艳勤, 王华, 黄维扬. 新型双极传输特性橙光铱(III)配合物的设计、合成及其电致发光★[J]. 化学学报, 2023, 81(8): 891-897. |
| [2] | 汪阳, 阎敬灵. 不同配体的稀土金属配合物在烯烃聚合领域的研究进展[J]. 化学学报, 2023, 81(3): 275-288. |
| [3] | 李波, 周海燕, 马海燕, 黄吉玲. 亚乙基桥联双茚锆、铪配合物的合成及催化丙烯选择性齐聚研究: 茚环3-位取代基的影响[J]. 化学学报, 2023, 81(10): 1280-1294. |
| [4] | 李志凯, 罗思琪, 陈敏, 於秀君, 李霄鹏. 双三联吡啶钌配合物的研究进展★[J]. 化学学报, 2023, 81(10): 1447-1461. |
| [5] | 马雪璐, 李蒙, 雷鸣. 三核过渡金属配合物在催化反应中的研究进展[J]. 化学学报, 2023, 81(1): 84-99. |
| [6] | 张琪, 江梦云, 刘天一, 曾意迅, 石胜伟. 可蒸镀自旋交叉配合物的薄膜与器件[J]. 化学学报, 2022, 80(9): 1351-1363. |
| [7] | 陈霄, 许汉华, 石向辉, 魏俊年, 席振峰. 稀土和锕系配合物促进的氮气活化与转化研究[J]. 化学学报, 2022, 80(9): 1299-1308. |
| [8] | 朱诗敏, 黄鑫, 韩勰, 刘思敏. N^C^N型Pt(II)配合物与大环主体葫芦[10]脲的识别及发光性质研究[J]. 化学学报, 2022, 80(8): 1066-1070. |
| [9] | 栾雪菲, 王聪芝, 夏良树, 石伟群. 铀酰与羧酸和肟基类配体相互作用的理论研究[J]. 化学学报, 2022, 80(6): 708-713. |
| [10] | 李斌, 于吉攀, 刘康, 吴群燕, 刘琦, 石伟群. 基于三脚架配体构筑的锕系-配体多重键的研究进展[J]. 化学学报, 2021, 79(8): 986-998. |
| [11] | 宋龙飞, 周妍妍, 高婷, 闫鹏飞, 李洪峰. 点手性调控的三股铕螺旋体的非对映选择性自组装及圆偏振发光[J]. 化学学报, 2021, 79(8): 1042-1048. |
| [12] | 赵添堃, 王鹏, 姬明宇, 李善家, 杨明俊, 蒲秀瑛. Salan钛双齿配合物的Sonogashira合成后修饰反应研究[J]. 化学学报, 2021, 79(11): 1385-1393. |
| [13] | 李金华, 卓庆德, 卓凯玥, 陈大发, 夏海平. 铱杂碳龙配合物的合成及反应性[J]. 化学学报, 2021, 79(1): 71-80. |
| [14] | 杨忠杰, 张小飞, 施亚男, 隆昶, 张彬灏, 闫书豪, 常琳, 唐智勇. 二维疏水铜基纳米片的合成及在硫醚类化合物催化氧化中的应用[J]. 化学学报, 2020, 78(9): 980-988. |
| [15] | 刘启雁, 蔡戴宏, 戚永育, 乐学义. 司帕沙星及均三嗪衍生物铜(II)配合物与DNA作用及其抗肿瘤活性[J]. 化学学报, 2020, 78(3): 263-270. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||