化学学报 ›› 2023, Vol. 81 ›› Issue (1): 1-5.DOI: 10.6023/A22110454 上一篇 下一篇
所属专题: 有机氟化学合集
研究通讯
杨春晖a, 陈景超a,*( ), 李新汉b, 孟丽b, 王凯民b, 孙蔚青b, 樊保敏a,b,*(
), 李新汉b, 孟丽b, 王凯民b, 孙蔚青b, 樊保敏a,b,*( )
)
投稿日期:2022-11-09
发布日期:2023-01-03
基金资助:
Chunhui Yanga, Jingchao Chena( ), Xinhan Lib, Li Mengb, Kaimin Wangb, Weiqing Sunb, Baomin Fana,b(
), Xinhan Lib, Li Mengb, Kaimin Wangb, Weiqing Sunb, Baomin Fana,b( )
)
Received:2022-11-09
Published:2023-01-03
Contact:
*E-mail: Supported by:文章分享
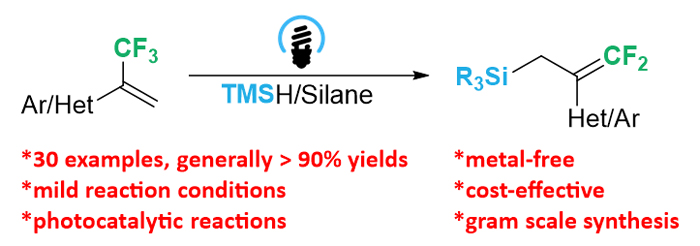
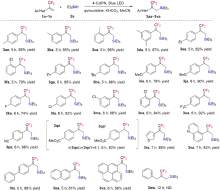
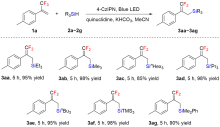
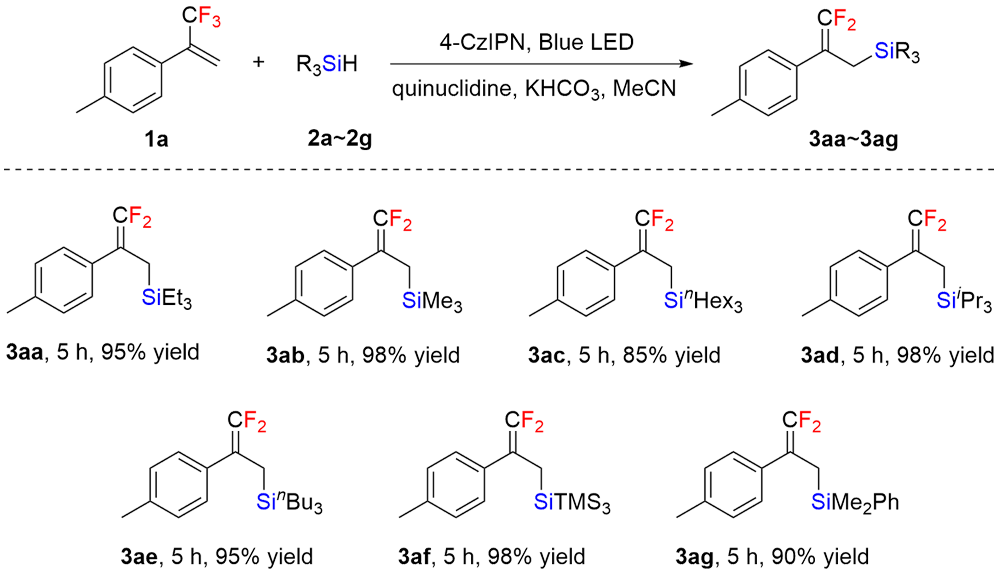
研究了在蓝光照射下, 以奎宁环作为氢原子转移试剂, 实现了硅烷与α-三氟甲基烯烃的无金属二氟烯丙基化反应, 并且通过使用多种芳香族和杂环α-三氟甲基烯烃, 均能成功得到二氟烯丙基硅烷. 本研究为制备二氟烯丙基硅烷提供了一种高效且经济的克级合成方法.
杨春晖, 陈景超, 李新汉, 孟丽, 王凯民, 孙蔚青, 樊保敏. 可见光催化的硅烷二氟烯丙基化反应[J]. 化学学报, 2023, 81(1): 1-5.
Chunhui Yang, Jingchao Chen, Xinhan Li, Li Meng, Kaimin Wang, Weiqing Sun, Baomin Fan. Difluoroallylation of Silanes under Photoirradiation[J]. Acta Chimica Sinica, 2023, 81(1): 1-5.
| Entry | Photocatalyst | Additive | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | quinuclidine | THF | NR |
| 2 | Eosin-Y | quinuclidine | THF | NR |
| 3 | DDQ | quinuclidine | THF | NR |
| 4 | Riboflavin | quinuclidine | THF | NR |
| 5 | Acridine Orange | quinuclidine | THF | NR |
| 6 | Ru(bpy)3Cl2 | quinuclidine | THF | NR |
| 7 | Methyl Orange | quinuclidine | THF | NR |
| 8 | 4-CzIPN | quinuclidine | THF | 80 (3aa') |
| 9 | 4-CzIPN | quinuclidine | MeCN | 85 |
| 10 | 4-CzIPN | quinuclidine | Toluene | ND |
| 11 | 4-CzIPN | quinuclidine | DMF | 23 |
| 12 | 4-CzIPN | quinuclidine | DCE | Trace |
| 13 | 4-CzIPN | quinuclidine | 1,4-dioxane | 21 |
| 14 | 4-CzIPN | DABCO | MeCN | NR |
| 15 | 4-CzIPN | Pyridine | MeCN | NR |
| 16 | 4-CzIPN | DIPEA | MeCN | NR |
| 17c | 4-CzIPN | quinuclidine | MeCN | 95 |
| 18c | — | quinuclidine | MeCN | NR |
| 19c | 4-CzIPN | — | MeCN | NR |
| 20c,d | 4-CzIPN | quinuclidine | MeCN | NR |
| Entry | Photocatalyst | Additive | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | quinuclidine | THF | NR |
| 2 | Eosin-Y | quinuclidine | THF | NR |
| 3 | DDQ | quinuclidine | THF | NR |
| 4 | Riboflavin | quinuclidine | THF | NR |
| 5 | Acridine Orange | quinuclidine | THF | NR |
| 6 | Ru(bpy)3Cl2 | quinuclidine | THF | NR |
| 7 | Methyl Orange | quinuclidine | THF | NR |
| 8 | 4-CzIPN | quinuclidine | THF | 80 (3aa') |
| 9 | 4-CzIPN | quinuclidine | MeCN | 85 |
| 10 | 4-CzIPN | quinuclidine | Toluene | ND |
| 11 | 4-CzIPN | quinuclidine | DMF | 23 |
| 12 | 4-CzIPN | quinuclidine | DCE | Trace |
| 13 | 4-CzIPN | quinuclidine | 1,4-dioxane | 21 |
| 14 | 4-CzIPN | DABCO | MeCN | NR |
| 15 | 4-CzIPN | Pyridine | MeCN | NR |
| 16 | 4-CzIPN | DIPEA | MeCN | NR |
| 17c | 4-CzIPN | quinuclidine | MeCN | 95 |
| 18c | — | quinuclidine | MeCN | NR |
| 19c | 4-CzIPN | — | MeCN | NR |
| 20c,d | 4-CzIPN | quinuclidine | MeCN | NR |

| [1] |
Chen, C.-A.; Sieburth, S. M.; Glekas, A.; Hewitt, G. W.; Trainor, G. L.; Erickson-Viitanen, S.; Garber, S. S.; Cordova, B.; Jeffry, S.; Klabe, R. M. Chem. Biol. 2001, 8, 1161.
doi: 10.1016/S1074-5521(01)00079-5 |
| [2] |
Mutahi, M. W.; Nittoli, T.; Guo, L.-X.; Sieburth, S. M. J. Am. Chem. Soc. 2002, 124, 7363.
doi: 10.1021/ja026158w |
| [3] |
Gately, S.; West, R. Drug Dev. Res. 2007, 68, 156.
doi: 10.1002/ddr.20177 |
| [4] |
Franz, A. K.; Wilson, S. O. J. Med. Chem. 2013, 56, 388.
doi: 10.1021/jm3010114 |
| [5] |
Tacke, R.; Popp, F.; Müller, B.; Theis, B.; Burschka, C.; Hamacher, A.; Kassack, M. U.; Schepmann, D.; Wünsch, B.; Jurva, U.; Wellner, E. ChemMedChem 2008, 3, 152.
doi: 10.1002/cmdc.200700205 |
| [6] |
For reviews and recent examples, see: (a) Wang, D.; Chen, D.-H. Acta Chim. Sinica 1990, 48, 516. (in Chinese)
pmid: 11848898 |
|
( 王东, 陈德恒, 化学学报, 1990, 48, 516).
pmid: 11848898 |
|
|
(b) Fleming, I.; Barbero, A.; Walter, D. Chem. Rev. 1997, 97, 2063.
pmid: 11848898 |
|
|
(c) Hiyama, T. J. Organomet. Chem. 2002, 653, 58.
doi: 10.1016/S0022-328X(02)01157-9 pmid: 11848898 |
|
|
(d) Chabaud, L.; James, P.; Landais, Y. Eur. J. Org. Chem. 2004, 15, 3173.
pmid: 11848898 |
|
|
(e) Komiyama, T.; Minami, Y.; Hiyama, T. ACS Catal. 2017, 7, 631.
doi: 10.1021/acscatal.6b02374 pmid: 11848898 |
|
| [7] |
For reviews and recent examples, see: (a) Tseng, C.-L.; Zhuo, R.-X.; Liu, J.-W. Acta Chim. Sinica 1964, 30, 360. (in Chinese)
pmid: 18767843 |
|
( 曾昭抡, 卓仁禧, 刘基万, 化学学报, 1964, 30, 360).
pmid: 18767843 |
|
|
(b) Chan, T. H.; Wang, D. Chem. Rev. 1995, 95, 1279.
doi: 10.1021/cr00037a007 pmid: 18767843 |
|
|
(c) Langkopf, E.; Schinzer, D. Chem. Rev. 1995, 95, 1375.
doi: 10.1021/cr00037a011 pmid: 18767843 |
|
|
(d) Barbero, A.; Pulido, F. J. Acc. Chem. Res. 2004, 37, 817.
doi: 10.1021/ar0400490 pmid: 18767843 |
|
|
(e) Nielsen, L.; Skrydstrup, T. J. Am. Chem. Soc. 2008, 130, 13145.
doi: 10.1021/ja804720p pmid: 18767843 |
|
|
(f) Hu, Y.-Q.; Huang, D.-F.; Wang, K.-H.; Zhao, Z.-X.; Zhao, F.-X.; Xu, W.-G.; Hu, Y.-L. Chin. J. Org. Chem. 2020, 40, 1689. (in Chinese)
doi: 10.6023/cjoc201912006 pmid: 18767843 |
|
|
虎永琴, 黄丹凤, 王克虎, 赵转霞, 赵芳霞, 徐炜刚, 胡雨来, 有机化学, 2020, 40, 1689).
doi: 10.6023/cjoc201912006 pmid: 18767843 |
|
| [8] |
For recent examples, see: (a) Murakami, K.; Yorimitsu, H.; Oshima, K. J. Org. Chem. 2009, 74, 1415.
doi: 10.1021/jo802433t pmid: 31524413 |
|
(b) Selander, N.; Paasch, J. R.; Szabó, K. J. J. Am. Chem. Soc. 2011, 133, 409.
doi: 10.1021/ja1096732 pmid: 31524413 |
|
|
(c) Vulovic, B.; Cinderella, A. P.; Watson, D. A. ACS Catal. 2017, 7, 8113.
doi: 10.1021/acscatal.7b03465 pmid: 31524413 |
|
|
(d) Larsson, J. M.; Szabó, K. J. J. Am. Chem. Soc. 2013, 135, 443.
doi: 10.1021/ja309860h pmid: 31524413 |
|
|
(e) Gan, Y.; Xu, W.; Liu, Y.-H. Org. Lett. 2019, 21, 9652.
doi: 10.1021/acs.orglett.9b03822 pmid: 31524413 |
|
|
(f) Yang, B.; Wang, Z.-X. Org. Lett. 2019, 21, 7965.
doi: 10.1021/acs.orglett.9b02946 pmid: 31524413 |
|
| [9] |
For recent examples, see: (a) Selander, N.; Paasch, J. R.; Szabó, K. J. J. Am. Chem. Soc. 2011, 133, 409.
doi: 10.1021/ja1096732 pmid: 31742415 |
|
(b) Larsson, J. M.; Szabó, K. J. J. Am. Chem. Soc. 2013, 135, 443.
doi: 10.1021/ja309860h pmid: 31742415 |
|
|
(c) Yang, B.; Wang, Z.-X. Org. Lett. 2019, 21, 7965.
doi: 10.1021/acs.orglett.9b02946 pmid: 31742415 |
|
|
(d) Gan, Y.; Xu, W.; Liu, Y.-H. Org. Lett. 2019, 21, 9652.
doi: 10.1021/acs.orglett.9b03822 pmid: 31742415 |
|
| [10] |
For recent examples, see: (a) Ohmura, T.; Taniguchi, H.; Suginome, M. J. Am. Chem. Soc. 2006, 128, 13682.
doi: 10.1021/ja063934h pmid: 27539673 |
|
(b) Miller, Z. D.; Li, W.; Belderrain, T. R.; Montgomery, J. J. Am. Chem. Soc. 2013, 135, 15282.
doi: 10.1021/ja407749w pmid: 27539673 |
|
|
(c) Miller, Z. D.; Dorel, R.; Montgomery, J. Angew. Chem. Int. Ed. 2015, 54, 9088.
doi: 10.1002/anie.201503521 pmid: 27539673 |
|
|
(d) Yeung, K.; Ruscoe, R. E.; Rae, J.; Pulis, A. P.; Procter, D. J. Angew. Chem. Int. Ed. 2016, 55, 11912.
doi: 10.1002/anie.201606710 pmid: 27539673 |
|
|
(e) Da, B.-C.; Liang, Q.-J.; Luo, Y.-C.; Ahmad, T.; Xu, Y.-H.; Loh, T.-P. ACS Catal. 2018, 8, 6239.
doi: 10.1021/acscatal.8b01547 pmid: 27539673 |
|
|
(f) Da, B.-C.; Liang, Q.-J.; Luo, Y.-C.; Ahmad, T.; Xu, Y.-H.; Loh, T.-P. ACS Catal. 2018, 8, 6239.
doi: 10.1021/acscatal.8b01547 pmid: 27539673 |
|
|
(g) Sang, H.-L.; Yu, S.-J.; Ge, S.-Z. Chem. Sci. 2018, 9, 973.
doi: 10.1039/C7SC04002D pmid: 27539673 |
|
|
(h) Zeng, J.-H.; Chen, J.-J.; Chen, L.; Zhan, Z.-P. Org. Chem. Front. 2020, 7, 1132.
doi: 10.1039/D0QO00156B pmid: 27539673 |
|
|
(i) Xu, J.-L.; Xu, Z.-Y.; Wang, Z.-L.; Ma, W.-W.; Sun, X.-Y.; Fu, Y.; Xu, Y.-H. J. Am. Chem. Soc. 2022, 144, 5535.
doi: 10.1021/jacs.2c00260 pmid: 27539673 |
|
| [11] |
(a) Takeda, M.; Shintani, R.; Hayashi, T. J. Org. Chem. 2013, 78, 5007.
doi: 10.1021/jo400888b pmid: 29202243 |
|
(b) Xiao, Y.-L.; Pan, Q.; Zhang, X.-G. Acta Chim. Sinica 2015, 73, 383. (in Chinese)
doi: 10.6023/A15010042 pmid: 29202243 |
|
|
( 肖玉兰, 潘强, 张新刚, 化学学报, 2015, 73, 383).
doi: 10.6023/A15010042 pmid: 29202243 |
|
|
(c) Mata, S.; López, L. A.; Vicente, R. Angew. Chem. Int. Ed. 2017, 56, 7930.
doi: 10.1002/anie.201703319 pmid: 29202243 |
|
|
(d) Hofstra, J. L.; Cherney, A. H.; Ordner, C. M.; Reisman, S. E. J. Am. Chem. Soc. 2018, 140, 139.
doi: 10.1021/jacs.7b11707 pmid: 29202243 |
|
|
(e) Zhao, S.; Li, C.-P.; Xu, B.; Liu, H. Chin. J. Org. Chem. 2020, 40, 1549. (in Chinese)
doi: 10.6023/cjoc202004039 pmid: 29202243 |
|
|
( 赵森, 李淳朴, 许斌, 柳红, 有机化学, 2020, 40, 1549).
doi: 10.6023/cjoc202004039 pmid: 29202243 |
|
| [12] |
(a) Yue, F.-Y.; Liu, J.-H.; Ma, H.-N.; Liu, Y.-X.; Dong, J.-Y.; Wang, Q.-M. Org. Lett. 2022, 24, 4019.
doi: 10.1021/acs.orglett.2c01448 |
|
(b) Luo, C.; Zhou, Y.; Chen, H.; Wang, T.; Zhang, Z.-B.; Han, P.; Jing, L.-H. Org. Lett. 2022, 24, 4286.
doi: 10.1021/acs.orglett.2c01690 |
|
|
(c) Xu, W.-G.; Xia, C.-J.; Shao, Q.; Zhang, Q.; Liu, M.-R.; Zhang, H.-W.; Wu, M.-B. Org. Chem. Front. 2022, 9, 4949.
doi: 10.1039/D2QO00894G |
|
| [13] |
(a) Zhou, Q.-Q.; Düsel, S. J. S.; Lu, L.-Q.; König, B.; Xiao, W.-J. Chem. Commun. 2019, 55, 107.
doi: 10.1039/C8CC08362B |
|
(b) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 |
|
| [14] |
(a) Li, K.-K.; Zhang, X.-X.; Chen, J.-C.; Gao, Y.; Yang, C.-H.; Zhang, K.-Y.; Zhou, Y.-Y.; Fan, B.-M. Org. Lett. 2019, 21, 9914.
doi: 10.1021/acs.orglett.9b03855 |
|
(b) Zhang, X.-X.; Chen, J.-C.; Gao, Y.; Li, K.-K.; Zhou, Y.-Y.; Sun, W.-Q.; Fan, B.-M. Org. Chem. Front. 2019, 6, 2410.
doi: 10.1039/C9QO00231F |
|
|
(c) Li, K.-K.; Chen, J.-C.; Yang, C.-H.; Zhang, K.-Y.; Pan, C.-X.; Fan, B.-M. Org. Lett. 2020, 22, 4261.
doi: 10.1021/acs.orglett.0c01294 |
|
|
(d) Lv, H.-P.; Laishram, R. D.; Chen, J.-C.; Khan, R.; Zhu, Y.-B.; Wu, S.-Y.; Zhang, J.-Q.; Liu, X.-Y.; Fan, B.-M. Chem. Commun. 2021, 57, 3660.
doi: 10.1039/D1CC00129A |
|
| [15] |
(a) Zhang, X.-H.; MacMillan, D. W. C. J. Am. Chem. Soc. 2017, 139, 11353.
doi: 10.1021/jacs.7b07078 |
|
(b) Le, C.; Liang, Y.-F.; Evans, R. W.; Li, X.-M.; MacMillan, D. W. C. Nature 2017, 547, 79.
doi: 10.1038/nature22813 |
|
|
(c) Hou, J.; Ee, A.; Cao, H.; Ong, H.-W.; Xu, J.-H.; Wu, J. Angew. Chem. Int. Ed. 2018, 57, 17220.
doi: 10.1002/anie.201811266 |
|
|
(d) Lei, G.-Y.; Xu, M.-C.; Chang, R.; Funes-Ardoiz, I.; Ye, J.-T. J. Am. Chem. Soc. 2021, 143, 11251.
doi: 10.1021/jacs.1c05852 |
| [1] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [2] | 吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156. |
| [3] | 刘嘉文, 林玮璜, 王惟嘉, 郭学益, 杨英. Cu1.94S-SnS纳米异质结的合成及其光催化降解研究[J]. 化学学报, 2023, 81(7): 725-734. |
| [4] | 何明慧, 叶子秋, 林桂庆, 尹晟, 黄心翊, 周旭, 尹颖, 桂波, 汪成. 卟啉基共价有机框架的光催化研究进展★[J]. 化学学报, 2023, 81(7): 784-792. |
| [5] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [6] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [7] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [8] | 齐学平, 王飞, 张健. 后合成法构筑钛基金属有机框架及其应用[J]. 化学学报, 2023, 81(5): 548-558. |
| [9] | 陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623. |
| [10] | 高杨, 张学鑫, 余金生, 周剑. α-手性三级叠氮化合物的不对称催化合成新进展★[J]. 化学学报, 2023, 81(11): 1590-1608. |
| [11] | 解众舒, 薛中鑫, 许子文, 李倩, 王洪宇, 李维实. 石墨相氮化碳的共轭交联修饰及其对可见光催化产氢性能的影响[J]. 化学学报, 2022, 80(9): 1231-1237. |
| [12] | 祁育, 章福祥. 太阳能光催化分解水制氢※[J]. 化学学报, 2022, 80(6): 827-838. |
| [13] | 舒恒, 包义德日根, 那永. CdS基纳米管光催化氧化5-羟甲基糠醛选择性生成2,5-呋喃二甲醛[J]. 化学学报, 2022, 80(5): 607-613. |
| [14] | 龚雪, 马新国, 万锋达, 段汪洋, 杨小玲, 朱进容. 二维单层MoSi2X4 (X=N, P, As)的电子结构及光学性质研究[J]. 化学学报, 2022, 80(4): 510-516. |
| [15] | 安攀, 张庆慧, 杨状, 武佳星, 张佳颖, 王雅君, 李宇明, 姜桂元. 双碳目标下太阳能制氢技术的研究进展[J]. 化学学报, 2022, 80(12): 1629-1642. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||