化学学报 ›› 2023, Vol. 81 ›› Issue (8): 898-904.DOI: 10.6023/A23040164 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究论文
投稿日期:2023-04-26
发布日期:2023-09-14
作者简介:基金资助:
Jianghao Luo, Haowen Ma, Jiehao Zhang, Wei Zhou( ), Qian Cai(
), Qian Cai( )
)
Received:2023-04-26
Published:2023-09-14
Contact:
*E-mail: weizhou88@jnu.edu.cn; caiqian@jnu.edu.cn
About author:Supported by:文章分享
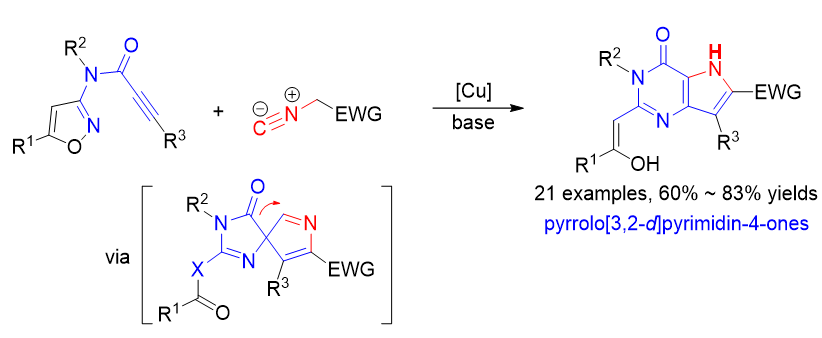
吡咯并[3,2-d]嘧啶-4-酮骨架在药物设计中广受关注, 该骨架是众多生物活性分子的核心结构单元. 目前已报道的吡咯并[3,2-d]嘧啶-4-酮骨架的合成方法有限且存在原料不易得、反应条件苛刻、合成步骤长、产率低和产物结构不易修饰等缺陷. 因此, 发展吡咯并[3,2-d]嘧啶-4-酮骨架的新型高效合成策略在合成化学与药物化学领域均具有重要的研究意义. 最近, 一种通过串联炔-异氰[3+2]环加成/Boulton-Katritzky重排/酰基迁移扩环反应构建9-去氮鸟嘌呤化合物的新方法被报道, 该方法以氮杂异噁唑衍生的炔丙酰胺化合物和异氰化合物为原料. 鉴于异噁唑化合物也是Boulton-Katritzky重排反应常使用的底物, 此工作进一步研究了一系列异噁唑衍生的炔丙酰胺化合物与异氰化合物的上述串联反应, 成功构建了一系列吡咯并[3,2-d]嘧啶-4-酮化合物.
罗江浩, 马浩文, 张杰豪, 周伟, 蔡倩. 串联炔-异氰[3+2]环加成/Boulton-Katritzky重排/扩环反应构建吡咯并[3,2-d]嘧啶-4-酮化合物★[J]. 化学学报, 2023, 81(8): 898-904.
Jianghao Luo, Haowen Ma, Jiehao Zhang, Wei Zhou, Qian Cai. Synthesis of Pyrrolo[3,2-d]pyrimidin-4-ones via Cascade Alkyne−isocyanide [3+2] Cycloaddition/Boulton-Katritzky Rearrangement/Ring Expansion Process★[J]. Acta Chimica Sinica, 2023, 81(8): 898-904.

| Entry | Catalyst | Solvent (V∶V) | Base | Yield of 3ab/% | Yield of 4ab/% | ||
|---|---|---|---|---|---|---|---|
| 1c | CuI | Tol/DMF (1∶3) | Cs2CO3 | Trace | 90 | ||
| 2d | CuI | Tol/DMF (1∶3) | Cs2CO3 | Trace | 88 | ||
| 3e | CuI | Tol/DMF (1∶3) | Cs2CO3 | 64 | 18 | ||
| 4 | CuI | Tol/DMF (1∶3) | Cs2CO3 | 79 | Trace | ||
| 5f | CuI | Tol/DMF (1∶3) | Cs2CO3 | 74 | Trace | ||
| 6 | CuBr | Tol/DMF (1∶3) | Cs2CO3 | 59 | Trace | ||
| 7 | Cu2O | Tol/DMF (1∶3) | Cs2CO3 | 46 | Trace | ||
| 8 | CuI | Tol | Cs2CO3 | 12 | 83 | ||
| 9 | CuI | DMF | Cs2CO3 | 67 | Trace | ||
| 10 | CuI | DMSO | Cs2CO3 | 61 | Trace | ||
| 11 | CuI | Tol/DMF (1∶3) | LiOtBu | 42 | Trace | ||
| 12 | CuI | Tol/DMF (1∶3) | NaOH | 48 | Trace | ||
| Entry | Catalyst | Solvent (V∶V) | Base | Yield of 3ab/% | Yield of 4ab/% | ||
|---|---|---|---|---|---|---|---|
| 1c | CuI | Tol/DMF (1∶3) | Cs2CO3 | Trace | 90 | ||
| 2d | CuI | Tol/DMF (1∶3) | Cs2CO3 | Trace | 88 | ||
| 3e | CuI | Tol/DMF (1∶3) | Cs2CO3 | 64 | 18 | ||
| 4 | CuI | Tol/DMF (1∶3) | Cs2CO3 | 79 | Trace | ||
| 5f | CuI | Tol/DMF (1∶3) | Cs2CO3 | 74 | Trace | ||
| 6 | CuBr | Tol/DMF (1∶3) | Cs2CO3 | 59 | Trace | ||
| 7 | Cu2O | Tol/DMF (1∶3) | Cs2CO3 | 46 | Trace | ||
| 8 | CuI | Tol | Cs2CO3 | 12 | 83 | ||
| 9 | CuI | DMF | Cs2CO3 | 67 | Trace | ||
| 10 | CuI | DMSO | Cs2CO3 | 61 | Trace | ||
| 11 | CuI | Tol/DMF (1∶3) | LiOtBu | 42 | Trace | ||
| 12 | CuI | Tol/DMF (1∶3) | NaOH | 48 | Trace | ||
| [1] |
(a) Bantia S.; Miller P. J.; Parker C. D.; Ananth S. L.; Horn L. L.; Kilpatrick J. M.; Morris P. E.; Hutchison T. L.; Montgomery J. A.; Sandhu J. S.; Int. Immunopharmacol. 2001, 1, 1199;
|
|
(b) Theoclitou M.-E.; Aquila, B.; Block, M. H.; Brassil, P. J.; Castriotta, L.; Code, E.; Collins, M. P.; Davies, A. M.; Deegan, T.; Ezhuthachan, J.; Filla, S.; Freed, E.; Hu, H.; Huszar, D.; Jayaraman, M.; Lawson, D.; Lewis, P. M.; Nadella, M. V. P.; Oza, V.; Padmanilayam, M.; Pontz, T.; Ronco, L.; Russell, D.; Whitston, D.; Zheng, X. J. Med. Chem. 2011, 54, 6734;
|
|
|
(c) Laufersweiler M. C.; Wang Y.; Soper D. L.; Suchanek M. K.; Fancher A. N.; Lu W.; Wang R. L.; Oppong K. A.; Ellis C. D.; Baize M. W.; O'Neil S. V.; Wos J. A.; Demuth T. P. Bioorg. Med. Chem. Lett. 2005, 15, 4322.
doi: 10.1016/j.bmcl.2005.06.046 |
|
| [2] |
(a) Semeraro T.; Lossani A.; Botta M.; Ghiron C.; Alvarez R.; Manetti F.; Mugnaini C.; Valensin S.; Focher F.; Corelli F.; J. Med. Chem. 2006, 49, 6037;
|
|
(b) Rodrigues M. V. N.; Barbosa, A. F.; da Silva, J. F.; dos Santos, D. A.; Vanzolini, K. L.; de Moraes, M. C.; Corrêa, A. G.; Cass, Q. B. Bioorg. Med. Chem. 2016, 24, 226;
|
|
|
(c) Tian C.; Wang, M.; Fang, F.; Zhang, Z.; Wang, X.; Liu, J. Eur. J. Med. Chem. 2017, 138, 630;
|
|
|
(d) Gangjee A.; Li, W.; Yang, J.; Kisliuk, R. L. J. Med. Chem. 2008, 51, 68;
|
|
|
(e) Huang L.; Li, H.; Li, L.; Niu, L.; Seupel, R.; Wu, C.; Cheng, W.; Chen, C.; Ding, B.; Brennan, P. E.; Yang, S. J. Med. Chem. 2019, 62, 4526;
|
|
|
(f) Zeng S.; Xie H.; Zeng L.-L.; Lu X.; Zhao X.; Zhang G.-C.; Tu Z.-C.; Xu H.-J.; Yang L.; Zhang X.-Q.; Hu W. Bioorg. Med. Chem. 2013, 21, 1749.
doi: 10.1016/j.bmc.2013.01.062 |
|
| [3] |
(a) Imai K.-I. Chem. Pharm. Bull. 1964, 12, 1030
doi: 10.1248/cpb.12.1030 |
|
(b) Taylor, E. C.; Young, W. B.; Ward, C. C. Tetrahedron Lett. 1993, 34, 4595;.
|
|
|
(c) Taylor, E. C.; Young, W. B. J. Org. Chem. 1995, 60, 7947;.
|
|
|
(d) Elliott, A. J.; Montgomery, J. A.; Walsh, D. A. Tetrahedron Lett. 1996, 37, 4339;.
|
|
|
(e) Furneaux, R. H.; Tyler, P. C. J. Org. Chem. 1999, 64, 8411;.
|
|
|
(f) Liu, M.-C.; Luo, M.-Z.; Mozdziesz, D. E.; Sartorelli, A. C. Synth. Commun. 2002, 32, 3797;.
|
|
|
(g) Elliott, A. J.; Morris, P. E.; Petty, Jr. S. L.; Williams, C. H. J. Org. Chem. 1997, 62, 8071;.
|
|
|
(h) Kwong, C. D.; Elliot, A. J.; Montgomery, J. A. J. Labelled Cpd. Radiopharm. 1998, 41, 879;.
|
|
|
(i) Shih H.; Cottam H. B.; Carson D. A. Chem. Pharm. Bull. 2002, 50, 364.
doi: 10.1248/cpb.50.364 |
|
| [4] |
(a) Boulton A. J.; Katritzky A. R.; Hamid A. M. J. Chem. Soc. C 1967, 2005;
|
|
(b) Afridi, A. S.; Katritzky, A. R.; Ramsden, C. A. J. Chem. Soc., Perkin Trans. 1 1976, 315;.
|
|
|
(c) For a book: Comprehensive Heterocyclic Chemistry II, Eds.: Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Elsevier, Amsterdam, 1996, Vols. 1-9.
|
|
| [5] |
For selected reviews and examples of rearrangement with isoxazoles and 1,2,4-oxadiazoles, see: (a) Pace, A.; Pierro, P.; Buscemi, S.; Vivona, N.; Barone, G. J. Org. Chem. 2009, 74, 351;
|
|
(b) Martorana, A.; Piccionello, A. P.; Buscemi, S.; Giorgi, G.; Pace, A. Org. Biomol. Chem. 2011, 9, 491;.
|
|
|
(c) Martorana, A.; Pace, A.; Buscemi, S.; Piccionello, A. P. Org. Lett. 2012, 14, 3240;.
|
|
|
(d) Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583;.
|
|
|
(e) Jones R. C. F.; Chatterley A.; Marty R.; Owton W. M.; Elsegood M. R. J. Chem. Commun. 2015, 51, 1112.
doi: 10.1039/C4CC07999J |
|
| [6] |
For selected reviews on ring expansion reactions, see: (a) Lu, B.-L.; Dai, L.; Shi, M. Chem. Soc. Rev. 2012, 41, 3318;
|
|
(b) Mack, D. J.; Njardarson, J. T. ACS Catal. 2013, 3, 272;
|
|
|
(c) Biletskyi, B.; Colonna, P.; Masson, K.; Parrain, J.-L.; Commeiras, L.; Chouraqui, G. Chem. Soc. Rev. 2021, 50, 7513;
|
|
|
(d) Li, D.; Zang, W.; Bird, M. J.; Hyland, C. J. T.; Shi, M. Chem. Rev. 2021, 121, 8685;
|
|
|
(e) Nanda, T.; Fastheem, M.; Linda, A.; Pati, B. V.; Banjare, S. K.; Biswal, P.; Ravikumar, P. C. ACS Catal. 2022, 12, 13247;
|
|
|
(f) Ye J. Chin. J. Org. Chem. 2021, 41, 1755.
doi: 10.6023/cjoc202100030 |
|
| [7] |
For selected examples on ring expansion reactions, see: (a) Luzung, M. R.; Markham, J. P.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 10858;
|
|
(b) Gorin, D. J.; Davis, N. R.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 11260;
|
|
|
(c) Chen, G.-Q.; Zhang, X. N.; Wei, Y.; Tang, X.-Y.; Shi, M. Angew. Chem., Int. Ed. 2014, 53, 8492;
|
|
|
(d) Pan, D.; Wei, Y.; Shi, M. Org. Lett. 2016, 18, 3930;
|
|
|
(e) Chen, G.-Q.; Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Chem. Sci. 2016, 7, 4318;
|
|
|
(f) Pan, D.; Wei, Y.; Shi, M. Org. Lett. 2017, 19, 3584;
|
|
|
(g) Wu, Q.-F.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 1680;
|
|
|
(h) Zhuo, C.-X.; Wu, Q.-F.; Zhao, Q.; Xu, Q.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 8169;
|
|
|
(i) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475;
|
|
|
(j) Wu, Q.; Zheng, C.; Zhuo, C.-X.; You, S.-L. Chem. Sci. 2016, 7, 4453;
|
|
|
(k) Wang, Y.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 15093;
|
|
|
(l) Zheng, C.; You, S.-L. Acc. Chem. Res. 2020, 53, 974;
|
|
|
(m) George, J.; Kim, H. Y.; Oh, K. Adv. Synth. Catal. 2016, 358, 3714;
|
|
|
(n) George, J.; Kim, H. Y.; Oh, K. Org. Lett. 2017, 19, 628;
|
|
|
(o) Ramu, G.; Ambala, S.; Nanubolu, J. B.; Babu, B. N. RSC Adv. 2019, 9, 35068;
|
|
|
(p) Ramu, G.; Tangella, Y.; Ambala, S.; Babu, B. N. J. Org. Chem. 2020, 85, 5370;
|
|
|
(q) Moisan, L.; Wagner, M.; Comesse, S.; Doris, E. Tetrahedron Lett. 2006, 47, 9093;
|
|
|
(r) Pesquet, A.; Daїch, L. Van Hijfte, J. Org. Chem. 2006, 71, 5303;
|
|
|
(s) Shang, S.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Angew. Chem., Int. Ed. 2014, 53, 6216;
|
|
|
(t) Guo, X.; Xing, Q.; Lei, K.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Adv. Synth. Catal. 2017, 359, 4393;
|
|
|
(u) Mamedov, V. A.; Mamedova, V. L.; Qu, Z.-W.; Zhu, H.; Galimullina, V. R.; Korshin, D. E.; Khikmatova, G. Z.; Litvinov, I. A.; Latypov, S. K.; Sinyashin, O. G.; Grimme, S. J. Org. Chem. 2021, 86, 13514;
|
|
|
(v) Mandal, S.; Pramanik, A. J. Org. Chem. 2022, 87, 9282;
|
|
|
(w) Zhu W.-K.; Xu L.-W. Chin. J. Org. Chem. 2023, 43, 362. (in Chinese)
doi: 10.6023/cjoc202300003 |
|
|
( 祝炜柯, 徐利文, 有机化学, 2023, 43, 362.)
|
|
| [8] |
For recent reviews on heterocyclic dearomatization: (a) Roche, S. P.; Porco, J. A. Angew. Chem., Int. Ed. 2011, 50, 4068;
|
|
(b) Donohoe, T. J.; Pullin, R. D. C. Chem. Commun. 2012, 48, 11924;
|
|
|
(c) Ding, Q.; Zhou, X.; Fan, R. Org. Biomol. Chem. 2014, 12, 4807;
|
|
|
(d) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc.Chem. Res. 2014, 47, 2558;
|
|
|
(e) Liang, X.-W.; Zheng, C.; You, S.-L. Chem. Eur. J. 2016, 22, 11918;
|
|
|
(f) Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164;
|
|
|
(g) Zheng, C.; You, S.-L. Chem 2016, 1, 830;
|
|
|
(h) Wu W.-T.; Zhang L.-M.; You S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese)
doi: 10.6023/A17020049 |
|
|
( 吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.)
|
|
| [9] |
Luo J.; Ma H.; Wu K.; Ao Y.; Zhou W.; Cai Q. Org. Lett. 2023, 25, 2123.
doi: 10.1021/acs.orglett.3c00575 |
| [10] |
For selected reviews on alkyne-isocyanide [3+2] cycloaddition, see: (a) Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajdenko, V. G. Chem. Rev. 2010, 110, 5235;
|
|
(b) Boyarskiy, V. P.; Bokach, N. A.; Luzyanin, K. V.; Kukushkin, V. Y. Chem. Rev. 2015, 115, 2698;
|
|
|
(c) Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295;
|
|
|
(d) Luo J.; Chen G.-S.; Chen S.-J.; Li Z.-D.; Liu Y.-L. Chem. Eur. J. 2021, 27, 6598.
doi: 10.1002/chem.v27.22 |
|
| [11] |
For selected examples, see: (a) Kamijo, S.; Kanazawa, C.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 9260;
|
|
(b) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664;
|
|
|
(c) Gao, M.; He, C.; Chen, H.; Bai, R.; Cheng, B.; Lei, A. Angew. Chem., Int. Ed. 2013, 52, 6958;
|
|
|
(d) Liu, J.; Fang, Z.; Zhang, Q.; Liu, Q.; Bi, X. Angew. Chem., Int. Ed. 2013, 52, 6953;
|
|
|
(e) Qi, X.; Zhang, H.; Shao, A.; Zhu, L.; Xu, T.; Gao, M.; Liu, C.; Lan, Y. ACS Catal. 2015, 5, 6640;
|
|
|
(f) Xiao, P.; Yuan, H.; Liu, J.; Zheng, Y.; Bi, X.; Zhang, J. ACS Catal. 2015, 5, 6177;
|
|
|
(g) Dong, J.; Bao, L.; Hu, Z.; Ma, S.; Zhou, X.; Hao, M.; Li, N.; Xu, X. Org. Lett. 2018, 20, 1244;
|
|
|
(h) He, X.-L.; Zhao, H.-R.; Song, X.; Jiang, B.; Du, W.; Chen, Y.-C.; ACS Catal. 2019, 9, 4374;
|
|
|
(i) Zheng, S.-C.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2019, 58, 1494;
|
|
|
(j) Liu, J.-Q.; Chen, X.; Shatskiy, A.; Kärkäs, M. D.; Wang, X.-S. J. Org. Chem. 2019, 84, 8998;
|
|
|
(k) Wang Y.; Zhou Y.; Song Q. Chem. Commun. 2020, 56, 6106.
doi: 10.1039/D0CC01919D |
|
| [12] |
Bird C. W. Tetrahedron 1985, 41, 1409.
doi: 10.1016/S0040-4020(01)96543-3 |
| [13] |
CCDC 2258385 (3j) contains the supplementary crystallographic data for this paper.
|
| [1] | 王瑞祥, 赵庆如, 顾庆, 游书力. 金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★[J]. 化学学报, 2023, 81(5): 431-434. |
| [2] | 邱孔茜, 李杰, 马浩文, 周伟, 蔡倩. 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023, 81(1): 42-63. |
| [3] | 杨妲, 张龙力, 刘欢, 杨朝合. 双功能配体修饰的Ir催化剂在“氢甲酰化-缩醛化”串联反应中的共催化作用[J]. 化学学报, 2021, 79(5): 658-662. |
| [4] | 许健, 张世樊, 罗莹, 张荔, 张帆, 黄挺菁, 宋秋玲. 自由基促进硫甲基取代的炔酮的环化反应[J]. 化学学报, 2019, 77(9): 932-938. |
| [5] | 姚坤, 刘浩, 袁乾家, 刘燕刚, 刘德龙, 张万斌. 钯催化三组分烯丙基串联反应: 化学专一性合成N-酰亚甲基-2-吡啶酮[J]. 化学学报, 2019, 77(10): 993-998. |
| [6] | 叶文波, 晏子聪, 万常峰, 侯豪情, 汪志勇. 一种新的肉桂酸类化合物的脱羧/甲基化反应[J]. 化学学报, 2018, 76(2): 99-102. |
| [7] | 宋颢, 刘小宇, 秦勇. 氮自由基化学新进展:光催化N-H键活化途径[J]. 化学学报, 2017, 75(12): 1137-1149. |
| [8] | 唐敏, 吴永, 刘源, 蔡茂强, 夏飞, 刘顺英, 胡文浩. “一锅法”不对称多组分串联反应合成手性氢化环氧异色烯衍生物:一种快速构建分子复杂性方法[J]. 化学学报, 2016, 74(1): 54-60. |
| [9] | 何玲, 顾梦迪, 王德先, 赵亮, 王梅祥. 三级烯酰胺的串联Heck反应——一种反式2,5-二芳基-3-吡咯啉化合物新颖的合成方法[J]. 化学学报, 2015, 73(10): 1018-1024. |
| [10] | 段德河, 殷勤, 王守国, 顾庆, 游书力. 手性磷酸催化的C(3)-取代吲哚和甲基乙烯基酮不对称串联反应[J]. 化学学报, 2014, 72(9): 1001-1004. |
| [11] | 耿浩兵, 陈珊珊, 孙玺, 张袖丽, 王磊. 铜催化2-(2,2-二溴乙烯基)苯酚化合物与苯酚衍生物的串联醚化反应[J]. 化学学报, 2014, 72(5): 595-601. |
| [12] | 李建晓, 汪朝阳, 薛福玲, 罗时荷. 含三唑结构的新型稠合三环2(5H)-呋喃酮衍生物的合成[J]. 化学学报, 2011, 69(23): 2835-2842. |
| [13] | 郭金波, 张淅芸, 陈庆华. 基于5-孟氧基-3-溴-2(5H)-呋喃酮的环丙烷合成方法研究:碳亲核试剂启动的不对称串联反应[J]. 化学学报, 2006, 64(19): 2008-2014. |
| [14] | 王大升,王道全,周长海. (±)-麝香酮和(R)-麝香酮的自由基扩环合成法[J]. 化学学报, 1995, 53(9): 909-915. |
| [15] | 田伟生,罗雍容,陈毓群,翟咏红. 从容易制备的三氟甲基恶唑酮直接合成三氟甲基吡咯及其有关化合物[J]. 化学学报, 1994, 52(11): 1126-1132. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||