化学学报 ›› 2025, Vol. 83 ›› Issue (9): 1025-1034.DOI: 10.6023/A25050158 上一篇 下一篇
研究展望
投稿日期:2025-05-11
发布日期:2025-06-23
作者简介: |
刘郅勍, 南方科技大学2021级本科生, 目前在刘柳课题组开展主族元素化学相关研究. |
 |
| 刘柳, 南方科技大学化学系研究员, 长期致力于双亲性主族元素化学的前沿探索, 聚焦亲核/亲电双功能主族元素分子体系的设计合成与转化研究, 并用其模拟过渡金属的电子结构和化学行为, 系统拓展主族元素的基础化学认知边界. 曾在2011年与2016年先后获得厦门大学学士、博士学位, 期间曾在加州大学圣地亚哥分校进行博士研究生联合培养. 随后赴多伦多大学和加州大学伯克利分校开展博士后研究(2016-2020), 2020年9月加入南方科技大学组建独立研究团队. 2021年入选国家级海外青年人才计划; 获得中国化学会—英国皇家化学会青年化学奖、中国化学会黄耀曾金属有机化学青年奖、中国化学会青年化学奖、中国化学会青委会菁青化学新锐奖、日本化学会杰出讲座奖、广东省青年科技创新奖. 目前担任《Inorganic Chemistry Frontiers》、《化学学报》、《EurJIC》、《Chinese Chemical Letters》青年编委. |
★ “中国青年化学家”专辑.
基金资助:Received:2025-05-11
Published:2025-06-23
Contact:
* E-mail: liuleoliu@sustech.edu.cn
About author:★ For the VSI “Rising Stars in Chemistry”.
Supported by:文章分享
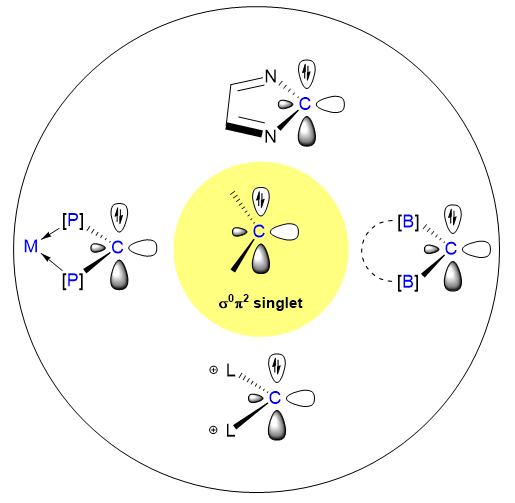
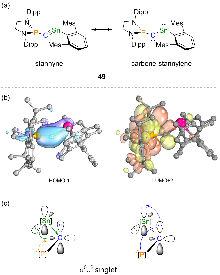
卡宾(R2C:)为一类中性二价碳物种, 其中心碳原子具有六电子结构特征, 在化学与材料科学等领域具有重要应用价值. 当前研究领域中, 已成功分离的稳定卡宾主要呈现σ2π0基态电子构型, 少量通过谱学表征的卡宾为σ1π1态; 而具有反转电子态(σ0π2)的稳定卡宾迄今仅一例报道. 该σ0π2卡宾具有一个刚性 RhP2C 四元金属杂环结构, 其中的卡宾碳原子在13C NMR中显示出低于-30的高场化学位移. 理论计算表明, 此类卡宾在小分子活化等过程中可能具有特殊反应活性. 本综述通过系统梳理该领域的研究进展, 重点分析其结构特征、稳定化策略及反应特性, 并展望其在惰性化学键活化、新型配体设计等方向的发展前景.
刘郅勍, 刘柳. 反转电子态(σ0π2)卡宾的发展及其研究展望★[J]. 化学学报, 2025, 83(9): 1025-1034.
Zhiqing Liu, Liu Leo Liu. Development and Outlook of Carbenes with Inverted Electronic Configuration (σ0π2)★[J]. Acta Chimica Sinica, 2025, 83(9): 1025-1034.








| [1] |
The Inorganic Chemistry Series, Group of Carbon, Silicon, and Germanium, Vol. 3, Science Press, Beijing, 1988 (in Chinese).
|
|
(无机化学丛书, 第三卷, 碳、硅、锗分族, 科学出版社, 北京, 1988.)
|
|
| [2] |
pmid: 11529717 |
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
(a)
|
|
(b)
|
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(黄家翩, 刘飞, 吴劼, 化学学报, 2023, 81, 520.)
doi: 10.6023/A23030088 |
|
|
(e)
|
|
|
(孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华, 化学学报, 2023, 81, 1271.)
doi: 10.6023/A23060268 |
|
|
(f)
|
|
|
(杜牧, 杨程博, 陈琦, 邓亮, 化学学报, 2024, 82, 932.)
doi: 10.6023/A24060203 |
|
| [14] |
(a)
doi: 10.1021/acs.chemrev.5b00220 pmid: 26391930 |
|
(b)
pmid: 26391930 |
|
|
(c)
pmid: 26391930 |
|
|
(于乐飞, 姚兴奇, 王剑波, 化学学报, 2023, 81, 1015.)
doi: 10.6023/A23050244 pmid: 26391930 |
|
|
(d)
pmid: 26391930 |
|
|
(任妍妍, 李欣, 韩英锋, 化学学报, 2023, 81, 735.)
doi: 10.6023/A23040130 pmid: 26391930 |
|
| [15] |
(a)
doi: 10.1039/c2dt32617e pmid: 23223752 |
|
(b)
pmid: 23223752 |
|
| [16] |
doi: 10.1021/cr940472u pmid: 11749234 |
| [17] |
|
| [18] |
|
| [19] |
|
|
(刘康, 李斌, 于吉攀, 石伟群, 化学学报, 2022, 80, 373.)
doi: 10.6023/A21120592 |
|
| [20] |
doi: 10.1021/ja407116e pmid: 24007553 |
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [36] |
doi: 10.1021/jacs.3c04933 pmid: 37354094 |
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
doi: 10.1021/ja066738j pmid: 17243835 |
| [41] |
doi: 10.1021/cr800549j pmid: 19368393 |
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
(a)
|
|
(b)
|
|
| [52] |
|
| [1] | 唐俊鸿, 周聪颖, 王成明. 氮杂环卡宾催化酰基肟生成亚胺自由基: 一种快速合成菲啶的方法[J]. 化学学报, 2025, 83(6): 557-562. |
| [2] | 杜牧, 杨程博, 陈琦, 邓亮. 氮杂环卡宾配位的铁磷簇合物[J]. 化学学报, 2024, 82(9): 932-939. |
| [3] | 郑剑, 林锦鸿, 肖吉昌. 基于二氟卡宾转化的芳基和烯基碘化物的三氟甲硫基化[J]. 化学学报, 2024, 82(2): 115-118. |
| [4] | 于乐飞, 姚兴奇, 王剑波. 重氮化合物在高分子合成化学中的应用进展★[J]. 化学学报, 2023, 81(8): 1015-1029. |
| [5] | 任妍妍, 李欣, 韩英锋. 基于氮杂环卡宾蓝光有机自由基的合成及其光学性质研究★[J]. 化学学报, 2023, 81(7): 735-740. |
| [6] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [7] | 李尚钊, 欧阳振武, 邹俊杰, 王东阳, 许斌, 邓亮. 氮杂环卡宾配位的单核型一价铁硫酚基配合物[J]. 化学学报, 2022, 80(3): 272-276. |
| [8] | 刘江, 徐敬成, Romana Pajkert, 梅海波, Gerd-Volker Röschenthaler, 韩建林. 光化学条件下(β-重氮-α,α-二氟乙基)膦酸酯与羧酸的酯化反应[J]. 化学学报, 2021, 79(6): 747-750. |
| [9] | 白云平, 崔春明. 硅卡宾铁(0)氮气配合物催化的炔烃选择性硼氢化反应[J]. 化学学报, 2020, 78(8): 763-766. |
| [10] | 凡一明, 程骏, 高亚飞, 施敏, 邓亮. 硼桥联三氮杂环卡宾配位的铁分子氮配合物:合成、表征和反应性质研究[J]. 化学学报, 2018, 76(6): 445-452. |
| [11] | 马星星, 轩晴晴, 宋秋玲. 含氮杂环的N—H和O—H二氟甲基化反应[J]. 化学学报, 2018, 76(12): 972-976. |
| [12] | 王乐明, 王骞, 陈杰安, 黄湧. Lewis酸对氮杂环卡宾协同催化体系中反应途径的调控[J]. 化学学报, 2018, 76(11): 850-856. |
| [13] | 李茂霖, 陈梦青, 徐彬, 朱守非, 周其林. 铑和手性螺环磷酸协同催化α-芳基重氮酮对醇的O—H键的不对称插入反应[J]. 化学学报, 2018, 76(11): 883-889. |
| [14] | 鲁鸿, 刘金宇, 李红玉, 许鹏飞. 氮杂环卡宾与过渡金属共催化反应研究进展[J]. 化学学报, 2018, 76(11): 831-837. |
| [15] | 李新玲, 王佳琪, 李龙, 尹应武, 叶龙武. 2H-吡咯的简易合成方法:金催化与路易斯酸催化的组合应用[J]. 化学学报, 2016, 74(1): 49-53. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
