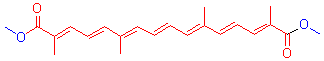
| [1] Fang, Z. K.; Wang, J. X. Nat. Prod. Res. Dev. 2007, 19, 280 (in Chinese). (方志凯, 王建新, 天然产物研究与开发, 2007, 19, 280.)[2] Li, N.; Lin, G.; Chiou, G. C. Y.; Min, Z. D. J. Chin. Pharm. Univ. 1999, 30, 108 (in Chinese). (李娜, 林鸽, Geroge C. Y. Chiou, 闵知大, 中国药科大学学报, 1999, 30, 108.)[3] Zhang, H.; Zhang, X. S.; Yan, F.; Zeng, Y. H.; Chen, F.; Wei, M. Chem. Res. Appl. 2000, 12, 487 (in Chinese). (张宏, 张新申, 颜钫, 曾宇红, 陈放, 魏明, 化学研究与应用, 2000, 12, 487.)[4] Zhang, H.; Zhang, J. G.; Zhang, L.; Yan, F.; Chen, F. Chem. Res. Appl. 2009, 21, 794 (in Chinese).(张宏, 张俊国, 张伶, 颜钫, 陈放, 化学研究与应用, 2009, 21, 794.)[5] Li, W. N.; Qian, Z. Y. Chin. J. New. Drugs 2005, 14, 1165 (in Chinese). (李文娜, 钱之玉, 中国新药杂志, 2005, 14, 1165.)[6] Zhang, J. G. M.S. Thesis, Sichuan Normal University, Chengdu, 2009, p. 27 (in Chinese). (张俊国, 硕士论文, 四川师范大学, 成都, 2009, p. 27.)[7] Zhang, J. G.; Zhang, H.; Zhang, L.; Yan, F.; Chen, F. Acta Chim. Sinica 2008, 66, 1451 (in Chinese). (张俊国, 张宏, 张伶, 颜钫, 陈放, 化学学报, 2008, 66, 1451.)[8] Yang, F.; Xu, B. Y.; Li, Q.; Zhao, K. Q.; Fan, Z. J. J. Atom Mol. Phys. 2010, 27, 605 (in Chinese). (杨帆, 徐布一, 李权, 赵可清, 范志金, 原子与分子物理学报, 2010, 27, 605.)[9] Liu, F. Y.; Hu, J. D.; Li, Q.; Zhao, K. Q. Chin. J. Org. Chem. 2007, 27, 507 (in Chinese). (刘焕英, 胡兢丹, 李权, 赵可清, 有机化学, 2007, 27, 507.)[10] Li, X. M.; Zhang, J. P. Acta Phys. Sin. 2010, 59, 7736 (in Chinese). (李雪梅, 张建平, 物理学报, 2010, 59, 7736.)[11] Wang, X. L. Organic Chemistry, Higher Education Press, Beijing, 2005, p. 4 (in Chinese). (汪小兰, 有机化学, 高等教育出版社, 北京, 2005, p. 4.)[12] Huang, H. S.; Zhang, T. L.; Zhang, J. G.; Wang, L. Q.; Yang, L.; Qiao, X. J.; Shang, J. Acta Chim. Sinica 2010, 68, 289 (in Chinese). (黄辉胜, 张同来, 张建国, 王丽琼, 杨利, 乔小晶, 尚静, 化学 学报, 2010, 68, 289.)[13] Fu, X. C.; Shen, W. X.; Yao, T. Y.; Hou, W. H. Physical Chemistry, Higher Education Press, Beijing, 2005 (in Chinese). (傅献彩, 沈文霞, 姚天扬, 侯文华, 物理化学, 高等教育出版 社, 北京, 2005.) |