有机化学 ›› 2021, Vol. 41 ›› Issue (2): 766-775.DOI: 10.6023/cjoc202007018 上一篇 下一篇
研究论文
席鑫1, 张公平1, 李建成1, 黄艳婷1, 蒋文军1, 吴鹏1, 朱红平1,*( )
)
收稿日期:2020-07-06
修回日期:2020-08-17
发布日期:2020-09-16
通讯作者:
朱红平
作者简介:基金资助:
Xin Xi1, Gongping Zhang1, Jiancheng Li1, Yanting Huang1, Wenjun Jiang1, Peng Wu1, Hongping Zhu1,*( )
)
Received:2020-07-06
Revised:2020-08-17
Published:2020-09-16
Contact:
Hongping Zhu
Supported by:文章分享

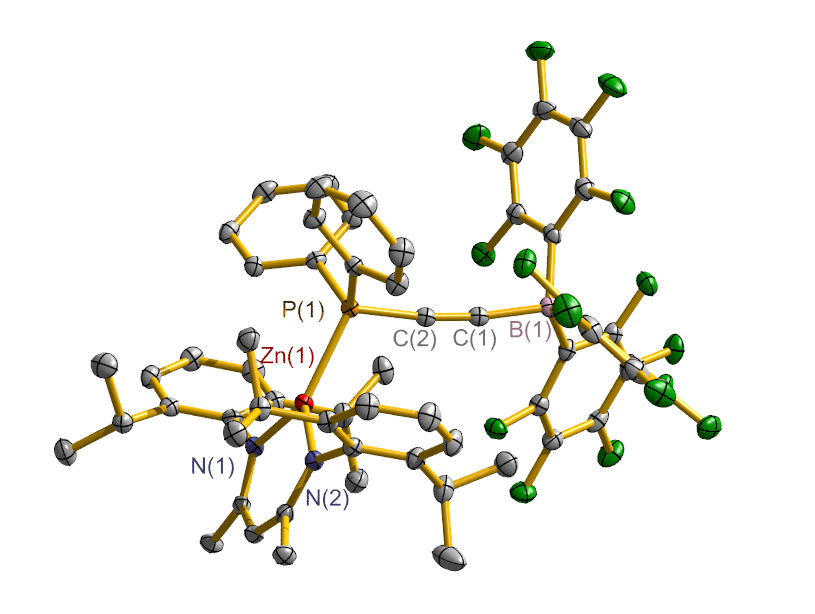
合成了5种不同取代基的炔类化合物Mes2HSiC≡CPh (1, Mes=2,4,6-Me3C6H2)、[tBuC(NAr)2]GeC≡CPh (2, Ar=2,6-iPr2C6H3)、[PhC(NtBu)2]SnC≡CPPh2 (3)、[HC(CMe)2(NAr)2]SnC≡CPPh2 (4)和[HC(CMe)2(NAr)2]ZnC≡CPPh2 (5), 研究了这些化合物与B(C6F5)3的反应. 在与B(C6F5)3的反应中, 1和2均发生1,1-碳硼化反应生成烯烃化合物(Ph)(Mes2HSi)C=C(C6F5)B(C6F5)2 (6)和{[tBuC(NAr)2]Ge}(Ph)C=C(C6F5)B(C6F5)2 (7), 7是一种GeII/B松散Lewis酸碱对化合物; 3~5则都发生B(C6F5)3与配体金属基的位置交换、进而配体金属基转换键合PPh2的反应, 分别生成新颖的分子内双性离子炔烃化合物[PhC(NtBu)2]SnP(Ph2)C≡CB(C6F5)3(8)、[HC(CMe)2(NAr)2]SnP(Ph2)C≡CB(C6F5)3 (9)、[HC(CMe)2(NAr)2]ZnP(Ph2)C≡CB(C6F5)3 (10). 文中还讨论了反应机理.
席鑫, 张公平, 李建成, 黄艳婷, 蒋文军, 吴鹏, 朱红平. 新型取代基炔类分子的合成及其与B(C6F5)3的反应[J]. 有机化学, 2021, 41(2): 766-775.
Xin Xi, Gongping Zhang, Jiancheng Li, Yanting Huang, Wenjun Jiang, Peng Wu, Hongping Zhu. Synthesis of Alkynes Composed of the Novel Substituents and Their Reactions with B(C6F5)3[J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 766-775.
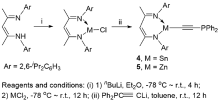

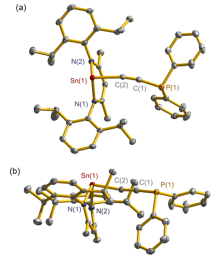
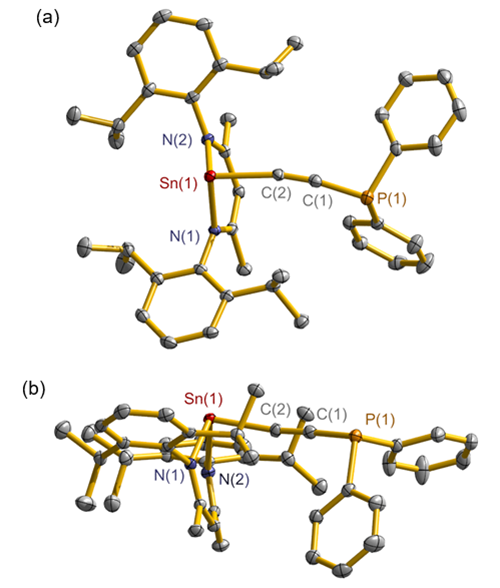
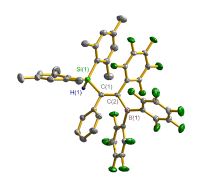
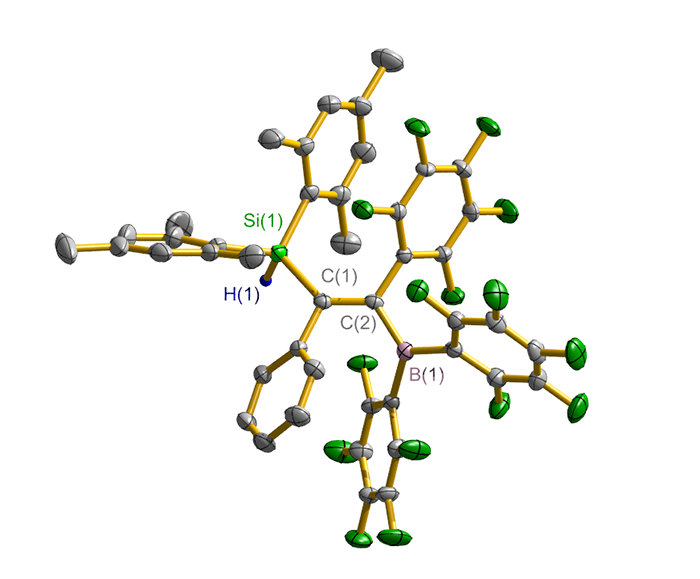
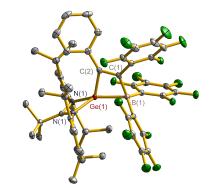
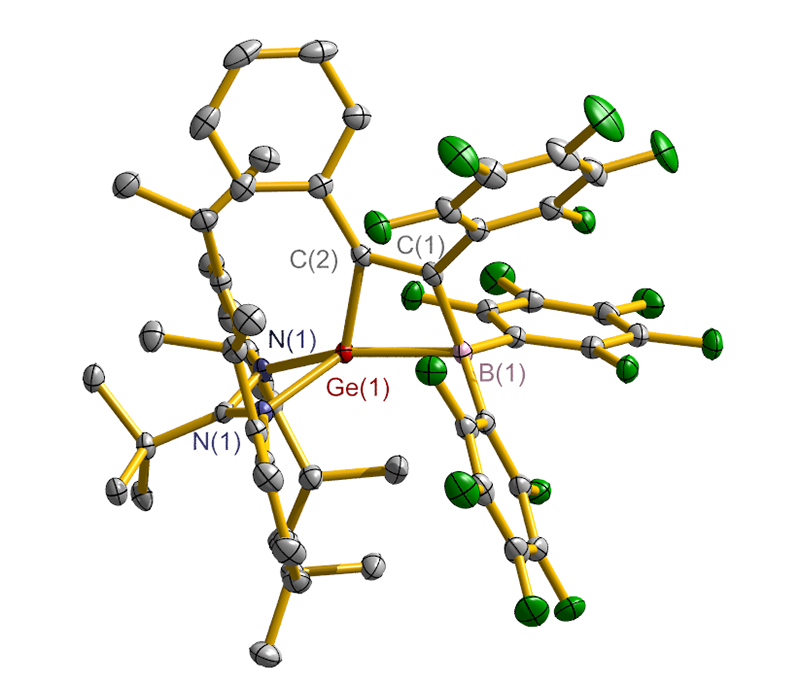
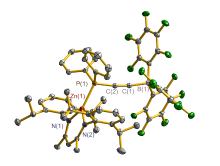
| [1] |
(a) Wrackmeyer B.; Zentgraf R. J. Chem. Soc., Chem. Commun. 1978, 402.
|
|
(b) Wrackmeyer B.; Bihlmayer C.; Schilling M. Chem. Ber. 1983, 116, 3182.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(c) Wrackmeyer B.; Dorfler U.; Kehr G.; Maisel H.E.; Milius W. J. Organomet. Chem. 1996, 524, 169.
doi: 10.1016/S0022-328X(96)06436-4 |
|
| [2] |
Parks D.J.; Piers W.E.; Yap G. P. A.Organometallics 1998, 17, 5492.
doi: 10.1021/om980673e |
| [3] |
Wrackmeyer B. Proc. 6th Int. Meet. on Boron Chemistry World Scientific, Singapore, 1987, 387.
|
|
(b) Wrackmeyer B. Coord. Chem. Rev. 1995, 145, 125.
|
|
|
(c) Wrackmeyer B. Heteroat. Chem. 2006, 17, 188.
doi: 10.1002/(ISSN)1098-1071 |
|
|
(d) Wrackmeyer B.; Khan E. Eur. J. Inorg. Chem. 2016, 300.
|
|
| [4] |
(a) Kehr G.; Erker G.Chem. Commun. 2012, 48, 1839.
doi: 10.1039/C1CC15628D |
|
(b) Kehr G.; Erker G.Chem. Sci. 2016, 7, 56.
doi: 10.1039/C5SC03282B |
|
| [5] |
Dierker G.; Ugolotti J.; Kehr G.; Fröhlich R.; Erker G. Adv. Synth. Catal. 2009, 351, 1080.
doi: 10.1002/adsc.v351:7/8 |
| [6] |
(a) Welch G.C.; San R.R.; Masuda J.D.; Stephan D. W.Science 2006, 314, 1124.
doi: 10.1126/science.1134230 |
|
(b) Stephan D.W. Acc. Chem. Res. 2015, 48, 306.
doi: 10.1021/ar500375j |
|
|
(c) Stephan D.W. J. Am. Chem. Soc. 2015, 137, 10018.
doi: 10.1021/jacs.5b06794 |
|
|
(d) Stephan D. W. Science 2016, 354, 1248.
|
|
|
(e) Stephan D.W.; Erker G. Angew. Chem., Int. Ed. 2015, 54, 6400.
doi: 10.1002/anie.201409800 |
|
| [7] |
Ekkert O.; Kehr G.; Fröhlich R.; Erker G. J. Am. Chem. Soc. 2011, 133, 4610.
doi: 10.1021/ja1110283 |
| [8] |
Eller C.; Kehr G.; Daniliuc C.G.; Fröhlich R.; Erker G. Organometallics 2013, 32, 384.
doi: 10.1021/om400016t |
| [9] |
(a) Tsao F.A.; Stephan D.W. Dalton Trans. 2015, 44, 71.
doi: 10.1039/c4dt03241a pmid: 25408099 |
|
(b) Tsao F.A.; Cao L.; Grimme S.; Stephan D.W. J. Am. Chem. Soc. 2015, 137, 13264.
doi: 10.1021/jacs.5b09526 pmid: 25408099 |
|
| [10] |
Li J.; Li B.; Liu R.; Jiang L.; Zhu H.; Roesky H.W.; Dutta S.; Koley D.; Liu W.; Ye Q. Chem. Eur. J. 2016, 22, 14499.
doi: 10.1002/chem.201603544 |
| [11] |
(a) Wrackmeyer B.; Horchler K.; Boese R. Angew. Chem. Int. Ed. 1989, 28, 1500.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Wrackmeyer B.; Kehr G.; Boese R. Chem. Ber. 1992, 125, 643.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(c) Wrackmeyer B.; Kehr G.; Sebald A.; Kummerlen J. Chem. Ber. 1992, 125, 1597.
doi: 10.1002/(ISSN)1099-0682 |
|
|
(d) Wettinger D. Inorg. Chim. Acta 1994, 220, 161.
doi: 10.1016/0020-1693(94)03869-4 |
|
|
(e) Ekkert O.; Kehr G.; Fröhlich R.; Erker G. Chem. Commun. 2011, 47, 10482.
doi: 10.1039/c1cc13008k |
|
| [12] |
Li J.; Wu P.; Jiang W.; Li B.; Wang B.; Zhu H.; Roesky H.W. Angew. Chem., Int. Ed. 2020,
doi: 10.1002/anie.202000899 |
| [13] |
Huang Y.; Wang X.; Li Y.; Yang M.-C.; Su M.-D; Zhu H. Chem. Commun. 2019, 55, 1494.
doi: 10.1039/C8CC09022J |
| [14] |
Powell S.A.; Tenenbaum J.M.; Woerpel K.A. J. Am. Chem. Soc. 2002, 124, 12648.
doi: 10.1021/ja027335w |
| [15] |
(a) Prust J.; Hohmeister H.; Stasch A.; Roesky H.W.; Magull J.; Alexopoulos E.; Usón I.; Schmidt H.; Noltemeyer M. Eur. J. Inorg. Chem. 2002, 2156.
|
|
(b) Dove A.P.; Gibson V.C.; Marshall E.L.; Rzepa H.S.; White A. J. P.; Williams D.J. J. Am. Chem. Soc. 2006, 128, 9834.
doi: 10.1021/ja061400a |
|
| [16] |
Jana A.; Roesky H.W.; Schulzke C.; Samuel P.P. Organometallics 2010, 29, 4837.
doi: 10.1021/om1000106 |
| [17] |
Liedtke R.; Kehr G.; Fröhlich R.; Daniliuc C.G.; Wibbeling B.; Petersen J.L.; Erker G. Helv. Chim. Acta 2012, 95, 2515.
doi: 10.1002/hlca.201200495 |
| [18] |
The Compile Group of the Practical Chemistry Handbook The Practical Chemistry Handbook, Science Press, Beijing, 2001. (in Chinese)
|
|
实用化学手册编写组, 实用化学手册, 科学出版社, 北京, 2001.).
|
|
| [19] |
Huang Y.; Jiang W.; Xi X.; Li Y.; Wang X.; Yang M.-C.; Zhang Z.-F.; Su M.-D; Zhu H. Eur. J. Inorg. Chem. 2020, 3496.
|
| [20] |
(a) Ding Y.; Hao H.; Roesky H.W.; Noltemeyer M.; Schmidt H.-G. Organometallics 2001, 20, 4806.
doi: 10.1021/om010358j |
|
(b) Rupar P.A.; Jennings M.C.; Ragogna P.J.; Baines K.M. Organometallics 2007, 26, 4109.
doi: 10.1021/om7006198 |
|
|
(c) Leung W.-P.; Chiu W.-K.; Mak T. C. W.Organometallics 2012, 31, 6966.
doi: 10.1021/om3007739 |
|
| [21] |
Wrackmeyer B. Annu. Rep. NMR Spectrosc. 1988, 20, 61.
|
| [22] |
Yu J.; Kehr G.; Daniliuc C.G.; Erker G. Inorg. Chem. 2013, 52, 11661.
doi: 10.1021/ic402139g |
| [23] |
Qian B.; Ward D.L.; Smith M.R. Organometallics 1998, 17, 3070.
doi: 10.1021/om970886o |
| [24] |
Green S.P.; Jones C.; Junk P.C.; Lippert K.A.; Stasch A. Chem. Commun. 2006, 3978.
|
| [25] |
Sen S.S.; Kritzler-Kosch M.; Nagendran S.; Roesky H.W. Eur. J. Inorg. Chem. 2010, 5304.
|
| [26] |
Massey A.G.; Park A.J. J. Organomet. Chem. 1964, 2, 245.
doi: 10.1016/S0022-328X(00)80518-5 |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||