有机化学 ›› 2021, Vol. 41 ›› Issue (2): 776-787.DOI: 10.6023/cjoc202007006 上一篇 下一篇
研究论文
丛婧a, 方芳a, 薛良敏a, 王锰a, 田超a, 王孝伟a, 刘俊义a,b, 张志丽a,*( )
)
收稿日期:2020-07-02
修回日期:2020-09-17
发布日期:2020-10-22
通讯作者:
张志丽
作者简介:基金资助:
Jing Conga, Fang Fanga, Liangmin Xuea, Meng Wanga, Chao Tiana, Xiaowei Wanga, Junyi Liua,b, Zhili Zhanga,*( )
)
Received:2020-07-02
Revised:2020-09-17
Published:2020-10-22
Contact:
Zhili Zhang
Supported by:文章分享
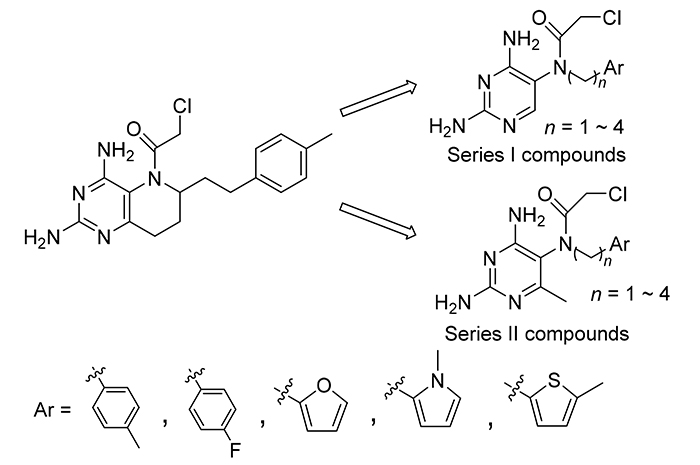
以课题组前期设计合成的非经典叶酸拮抗剂6-(4'-甲基苯乙基)-N5-氯乙酰基-2,4-二氨基哌啶并[3,2-d]嘧啶(wm-8.2)为先导化合物, 将wm-8.2中的哌啶并嘧啶双环结构简化为嘧啶单环结构, 以提高分子柔韧性并简化分子结构, 根据6-位空间占位设计6-H和6-甲基两个系列, 考察了不同桥链长度和不同芳香杂环侧链对抗肿瘤活性的影响. 同时对具有叶酸抑制剂分子结构特征的关键中间体进行活性对比测定, 研究了N(5)位氯乙酰基对活性的影响. 两个系列目标化合物和关键中间体共36个化合物的结构均经1H NMR, 13C NMR和MS确证. 生物活性测定表明, 6位为甲基的化合物中, 具有三碳桥链及对甲基苯环侧链的6-甲基-2,4-二氨基-5-(N-(4-甲基苯基)丙基-N-(2-氯乙酰基))氨基嘧啶(6b-3)具有最好的HL-60、A549和HCT116细胞增殖抑制活性, IC50分别为0.25, 0.83和0.63 μmol?L –1. 化合物6b-3在N(5)位氯乙酰基取代之前的关键中间体6-甲基-2,4-二氨基-5-(N-(4-甲基苯基)丙基)氨基嘧啶(5b-3)具有最优的二氢叶酸还原酶抑制活性. 总结了化合物的构效关系, 并用计算机模拟进行了阐释.
丛婧, 方芳, 薛良敏, 王锰, 田超, 王孝伟, 刘俊义, 张志丽. 新型嘧啶单环类非经典叶酸拮抗剂的合成及抗肿瘤活性研究[J]. 有机化学, 2021, 41(2): 776-787.
Jing Cong, Fang Fang, Liangmin Xue, Meng Wang, Chao Tian, Xiaowei Wang, Junyi Liu, Zhili Zhang. Synthesis and Antitumor Activity of Novel Pyrimidine Monocyclic Nonclassical Antifolates[J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 776-787.
| Compd. | n | IC50/(μmol?L–1)a | ||
|---|---|---|---|---|
| A549 | HCT-116 | HL-60 | ||
| 6a-1 | 1 | 10.44±3.29 | 3.72±0.08 | 5.24±0.34 |
| 6a-2 | 2 | 2.93±0.35 | 2.37±0.03 | 1.25±0.25 |
| 6a-3 | 3 | 2.62±0.42 | 0.98±0.34 | 0.60±0.01 |
| 6a-4 | 4 | 2.42±0.32 | 2.16±0.20 | 3.24±0.02 |
| 6b-1 | 1 | 18.35±2.45 | 3.88±0.24 | 1.65±0.23 |
| 6b-2 | 2 | 2.18±0.34 | 1.47±0.23 | 0.54±0.03 |
| 6b-3 | 3 | 0.83±0.13 | 0.63±0.08 | 0.25±0.08 |
| 6b-4 | 4 | 2.42±0.32 | 1.19±0.20 | 0.84±0.10 |
| 6a-5 | 2 | 8.24±2.45 | 1.57±0.35 | 0.92±0.23 |
| 6a-6 | 3 | 3.20±0.35 | 1.04±0.09 | 1.03±0.34 |
| 6b-5 | 2 | 2.34±0.23 | 0.99±0.39 | 1.43±0.43 |
| 6b-6 | 3 | 2.31±0.23 | 0.86±0.16 | 1.12±0.22 |
| 6a-7 | 1 | 29.98±0.97 | 9.32±0.93 | 10.99±0.99 |
| 6a-8 | 1 | 31.26±0.86 | 14.23±1.32 | 19.44±2.14 |
| 6a-9 | 1 | 48.46±3.21 | 9.83±0.92 | 34.24±2.15 |
| 6b-7 | 1 | 19.33±3.45 | 3.23±0.23 | 8.17±0.1 |
| 6b-8 | 1 | 26.42±2.26 | 10.33±0.89 | 8.93±0.94 |
| 6b-9 | 1 | 43.41±4.49 | 4.24±0.99 | 14.98±2.88 |
| 5a-1 | 1 | >100 | >100 | 74.10±2.14 |
| 5a-2 | 2 | 28.04±0.42 | >100 | 17.37±0.98 |
| 5a-3 | 3 | 18.51±0.44 | 29.40±3.87 | 12.68±0.97 |
| 5a-4 | 4 | 16.64±1.61 | >100 | 15.95±0.35 |
| 5b-1 | 1 | 61.26±1.72 | >100 | 14.59±1.12 |
| 5b-2 | 2 | 3.64±0.58 | 10.23+0.55 | 3.31±0.02 |
| 5b-3 | 3 | 4.00±0.33 | 14.18±1.69 | 3.72±0.82 |
| 5b-4 | 4 | 7.10±1.04 | >100 | 6.83±0.98 |
| 5a-5 | 2 | 19.24±0.98 | 34.26±1.24 | 38.24±0.23 |
| 5a-6 | 3 | 20.24±0.25 | 36.24±3.52 | 23.25±0.72 |
| 5b-5 | 2 | 10.24±0.24 | 19.43±0.48 | 13.53±0.92 |
| 5b-6 | 3 | 6.24±0.22 | 15.42±0.29 | 10.09±0.94 |
| 5a-7 | 1 | 18.42±0.24 | 25.26±0.92 | 32.42±2.58 |
| 5a-8 | 1 | 29.35±0.24 | 33.35±0.75 | 12.45±1.31 |
| 5a-9 | 1 | 23.25±2.34 | 28.45±2.32 | 23.12±2.15 |
| 5b-7 | 1 | 34.24±2.59 | 20.24±0.14 | 8.18±0.18 |
| 5b-8 | 1 | 43.25±0.33 | 36.25±0.96 | 14.24±0.34 |
| 5b-9 | 1 | 38.24±0.97 | 21.82±0.88 | 9.44±0.02 |
| MTX | 9.04±0.46 | 7.12±0.58 | 0.04±0.01 | |
| wm-8.2 | 0.28±0.03 | 0.82±0.02 | 1.08±0.03 | |
| Compd. | n | IC50/(μmol?L–1)a | ||
|---|---|---|---|---|
| A549 | HCT-116 | HL-60 | ||
| 6a-1 | 1 | 10.44±3.29 | 3.72±0.08 | 5.24±0.34 |
| 6a-2 | 2 | 2.93±0.35 | 2.37±0.03 | 1.25±0.25 |
| 6a-3 | 3 | 2.62±0.42 | 0.98±0.34 | 0.60±0.01 |
| 6a-4 | 4 | 2.42±0.32 | 2.16±0.20 | 3.24±0.02 |
| 6b-1 | 1 | 18.35±2.45 | 3.88±0.24 | 1.65±0.23 |
| 6b-2 | 2 | 2.18±0.34 | 1.47±0.23 | 0.54±0.03 |
| 6b-3 | 3 | 0.83±0.13 | 0.63±0.08 | 0.25±0.08 |
| 6b-4 | 4 | 2.42±0.32 | 1.19±0.20 | 0.84±0.10 |
| 6a-5 | 2 | 8.24±2.45 | 1.57±0.35 | 0.92±0.23 |
| 6a-6 | 3 | 3.20±0.35 | 1.04±0.09 | 1.03±0.34 |
| 6b-5 | 2 | 2.34±0.23 | 0.99±0.39 | 1.43±0.43 |
| 6b-6 | 3 | 2.31±0.23 | 0.86±0.16 | 1.12±0.22 |
| 6a-7 | 1 | 29.98±0.97 | 9.32±0.93 | 10.99±0.99 |
| 6a-8 | 1 | 31.26±0.86 | 14.23±1.32 | 19.44±2.14 |
| 6a-9 | 1 | 48.46±3.21 | 9.83±0.92 | 34.24±2.15 |
| 6b-7 | 1 | 19.33±3.45 | 3.23±0.23 | 8.17±0.1 |
| 6b-8 | 1 | 26.42±2.26 | 10.33±0.89 | 8.93±0.94 |
| 6b-9 | 1 | 43.41±4.49 | 4.24±0.99 | 14.98±2.88 |
| 5a-1 | 1 | >100 | >100 | 74.10±2.14 |
| 5a-2 | 2 | 28.04±0.42 | >100 | 17.37±0.98 |
| 5a-3 | 3 | 18.51±0.44 | 29.40±3.87 | 12.68±0.97 |
| 5a-4 | 4 | 16.64±1.61 | >100 | 15.95±0.35 |
| 5b-1 | 1 | 61.26±1.72 | >100 | 14.59±1.12 |
| 5b-2 | 2 | 3.64±0.58 | 10.23+0.55 | 3.31±0.02 |
| 5b-3 | 3 | 4.00±0.33 | 14.18±1.69 | 3.72±0.82 |
| 5b-4 | 4 | 7.10±1.04 | >100 | 6.83±0.98 |
| 5a-5 | 2 | 19.24±0.98 | 34.26±1.24 | 38.24±0.23 |
| 5a-6 | 3 | 20.24±0.25 | 36.24±3.52 | 23.25±0.72 |
| 5b-5 | 2 | 10.24±0.24 | 19.43±0.48 | 13.53±0.92 |
| 5b-6 | 3 | 6.24±0.22 | 15.42±0.29 | 10.09±0.94 |
| 5a-7 | 1 | 18.42±0.24 | 25.26±0.92 | 32.42±2.58 |
| 5a-8 | 1 | 29.35±0.24 | 33.35±0.75 | 12.45±1.31 |
| 5a-9 | 1 | 23.25±2.34 | 28.45±2.32 | 23.12±2.15 |
| 5b-7 | 1 | 34.24±2.59 | 20.24±0.14 | 8.18±0.18 |
| 5b-8 | 1 | 43.25±0.33 | 36.25±0.96 | 14.24±0.34 |
| 5b-9 | 1 | 38.24±0.97 | 21.82±0.88 | 9.44±0.02 |
| MTX | 9.04±0.46 | 7.12±0.58 | 0.04±0.01 | |
| wm-8.2 | 0.28±0.03 | 0.82±0.02 | 1.08±0.03 | |





| [1] |
Raz S.; Stark M.; Assaraf Y.G. Drug Resist. Updates 2016, 28, 43.
doi: 10.1016/j.drup.2016.06.004 |
| [2] |
Lilah R.; Ilan I.; Yotam K.; David G.P.; Gerrit J.; Yehuda G.A. Biochem. J. 2002, 367, 741.
doi: 10.1042/bj20020801 |
| [3] |
Al-Omary F. A. M.; Hassan G.S.; El-Messery S.M.; Nagi M.N.; El-Subbagh H.I. Eur. J. Med. Chem. 2017, 33, 335.
|
| [4] |
Wang M.; Tian C.; Xue L.M.; Li H.; Cong J.; Fang F.; Yang J.J.; Yuan M.M.; Chen Y.; Guo Y.; Wang X.W.; Liu J.Y.; Zhang Z.L. Eur. J. Med. Chem. 2020, 190, 112113.
doi: 10.1016/j.ejmech.2020.112113 |
| [5] |
Li H.; Fang F.; Liu Y.Q.; Xue L.M.; Wang M.; Guo Y.; Wang X.W.; Tian C.; Liu J.Y.; Zhan g, Z. L. Bioorg. Med. Chem. 2018, 26, 2674.
doi: 10.1016/j.bmc.2018.04.035 |
| [6] |
Ellis P.A.; Norman A.; Hill A.; O'BrienM M. E. R.; Nicolson M.; Hickish T.; Cunningham D. Eur. J. Cancer 1995, 31, 1594.
doi: 10.1016/0959-8049(95)00323-B |
| [7] |
Grant S. Adv. Cancer Res. 1997, 72, 197.
|
| [8] |
Wang M.; Yang J.J.; Yuan M.M.; Xue L.M.; Li H.; Tian C.; Wang X.W.; Liu J.Y.; Zhang Z.L. Eur. J. Med. Chem. 2017, 128, 88.
doi: 10.1016/j.ejmech.2017.01.033 |
| [9] |
Fang F.; Xue L.M.; Cong J.; Tian C.; Wang X.W.; Liu J.Y.; Zhang Z.L. Chem. J. Chin. Univ. 2019, 40, 844. (in Chinese)
|
|
方芳, 薛良敏, 丛婧, 田超, 王孝伟, 刘俊义, 张志丽, 高学校化学学报, 2019, 40, 844.).
|
|
| [10] |
Abdullah Z; Bakar M.A. J. Chem. Sci. 2006, 4, 241.
|
| [11] |
Weix D.J.; Dreher S.D.; Katz T.J. J. Am. Chem. Soc. 2000, 122, 10027.
doi: 10.1021/ja001904n |
| [12] |
Houjeiry T.I.; Poe S.L.; Mcquade D.T. Org. Lett. 2012, 14, 4394.
doi: 10.1021/ol301874x |
| [13] |
Gao T.F.; Zhang C.Y.; Shi X.W.; Guo R.; Zhang K.; Gu J.M.; Li L.; Li S.L.; Zheng Q.Q.; Cui M.Y.; Cui M.; Gao X.M.; Liu Y.; Wang L. Eur. J. Med. Chem. 2019, 178, 329.
doi: 10.1016/j.ejmech.2019.06.013 |
| [14] |
El-Subbagh H.I.; Hassan G.S.; El-Messery S.M.; Al-Rashood S.T.; Al-Omary F. A. M.; Abulfadl Y.S.; Shabayek M.I. Eur. J. Med. Chem. 2014, 74, 234.
doi: 10.1016/j.ejmech.2014.01.004 pmid: 24469112 |
| [15] |
Ng H.L.; Chen S.Y.; Chew E.H.; Chui W.K. Eur. J. Med. Chem. 2016, 115, 63.
doi: 10.1016/j.ejmech.2016.03.002 |
| [16] |
Zhang Z.L.; Tian C.; Zhou S.X.; Wang W.; Guo Y.; Xia J.; Liu Z.M.; Wang B.; Wang X.W.; Golding B.T.; Griff R.J.; Du Y.S.; Liu J.Y. Eur. J. Med. Chem. 2012, 58, 228.
doi: 10.1016/j.ejmech.2012.09.027 |
| [17] |
Sadanandam P.; Jyothi V.; Chari M.A.; Das P. Tetrahedron Lett. 2011, 52, 5521.
doi: 10.1016/j.tetlet.2011.08.076 |
| [18] |
Landor P.D.; Rydon H.N. J. Chem. Soc. 1955, 11, 1113.
|
| [19] |
Taylor E.; Barton J. J. Org. Chem. 1959, 24, 127.
doi: 10.1021/jo01083a619 |
| [20] |
Oki M.; Lwamura H.; Onoda T.; Lwamura M. Bull. Chem. Soc. Jpn. 1959, 32, 1135.
doi: 10.1246/bcsj.32.1135 |
| [21] |
An J.; Work D.N.; Kenyon C.; Procter D.J. J. Org. Chem. 2014, 79, 6743.
doi: 10.1021/jo501093g |
| [22] |
Jackman L.M.; Haddon V.R. J. Am. Chem. Soc. 1974, 96, 5130.
doi: 10.1021/ja00823a020 |
| [23] |
Solabannavar S.B.; Desai U.V.; Mane R.B. Indian J. Chem., Sect. B : Org. Chem. Incl. Med. Chem. 2004, 43, 2235.
|
| [24] |
Larionov E.; Lin L.; Guénée L.; Mazet C. J. Am. Chem. Soc. 2014, 136, 16882.
doi: 10.1021/ja508736u |
| [25] |
Bert L. Compt. Rend. Acad. Sci. Paris. 1928, 186, 699.
|
| [26] |
Kampmeier J.A.; Harris S.H.; Wedegaertner D.K. J. Org. Chem. 1980, 45, 315.
doi: 10.1021/jo01290a023 |
| [27] |
Bhatt V.; Samant S.D.; Pednekar, S. Synth. Commun. 2017, 47, 968.
|
| [28] |
Keith B.; Andrew C.B. J. Chem. Res., Miniprint 1992, 2, 514.
|
| [29] |
Gangjee A.; Jain H.D.; Queener S.F.; Kisliuk R.L. J. Med. Chem. 2008, 51, 4589.
doi: 10.1021/jm800244v |
| [1] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [2] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [3] | 王锋, 陈钰, 裴鸿艳, 张静, 张立新. 含哌啶的新型1,2,4-噁二唑类衍生物的设计合成及抗真菌活性研究[J]. 有机化学, 2023, 43(8): 2826-2836. |
| [4] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [5] | 张维舒, 聂礼飞, Khurshed Bozorov, 阿吉艾克拜尔•艾萨, 赵江瑜. 2,5-二氨基噻吩-3,4-二羧酸二乙酯衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(7): 2543-2552. |
| [6] | 庞盼杏, 宁蓉, 祝创, 黄文洁, 马献力, 蒋彩娜, 李芳耀, 周小群. 苦参碱缩氨基脲类化合物的合成及其体外抗肿瘤活性研究[J]. 有机化学, 2023, 43(6): 2126-2135. |
| [7] | 段康慧, 唐俊龙, 伍婉卿. 稠杂环化合物的合成及其抗肿瘤活性研究进展[J]. 有机化学, 2023, 43(3): 826-854. |
| [8] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [9] | 孙昌兴, 张福豪, 张欢, 李鹏辉, 姜林. 新型2-(1-甲基-1H-吡唑-4-基)嘧啶-4-甲酰胺的设计、合成、杀菌活性及分子对接研究[J]. 有机化学, 2023, 43(1): 229-235. |
| [10] | 刘威琴, 邵利辉, 李成朋, 邹雅玉, 龙海洮, 李焱, 戈强胜, 王贞超, 欧阳贵平. 3-腙喹唑啉酮衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(1): 214-222. |
| [11] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| [12] | 薛露, 张丽花, 张成玉, 赵鑫, 党伟帆, 王昭昕, 王春华, 所同川, 颜晓晖. 链霉菌CB03234-S中天赐霉素衍生物的发现[J]. 有机化学, 2022, 42(4): 1241-1247. |
| [13] | 王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883. |
| [14] | 孔媛芳, 杨彬, 庄严, 张京玉, 孙德梅, 董春红. 基于二肽基肽酶4 (DPP-4)靶点设计的五种降糖活性杂环合成及构效关系研究进展[J]. 有机化学, 2022, 42(3): 770-784. |
| [15] | 曾艳, 聂礼飞, 牛超, 阿依提拉•麦麦提江, Khurshed Bozorov, 赵江瑜, 阿吉艾克拜尔•艾萨. 二氢噁唑并[5,4-d]吡咯并[1,2-a]嘧啶酮的合成及生物活性研究[J]. 有机化学, 2022, 42(2): 543-556. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||