有机化学 ›› 2021, Vol. 41 ›› Issue (7): 2774-2787.DOI: 10.6023/cjoc202101028 上一篇 下一篇
研究论文
杨朝凯a, 武霞a, 张晋陆a, 路星星a, 李想a, 蒋志洋a, 宋敦伦b, 段红霞a,*( ), 杨新玲a
), 杨新玲a
收稿日期:2021-01-18
修回日期:2021-03-16
发布日期:2021-04-12
通讯作者:
段红霞
基金资助:
Zhaokai Yanga, Xia Wua, Jinlu Zhanga, Xingxing Lua, Xiang Lia, Zhiyang Jianga, Dunlun Songb, Hongxia Duana( ), Xinling Yanga
), Xinling Yanga
Received:2021-01-18
Revised:2021-03-16
Published:2021-04-12
Contact:
Hongxia Duan
Supported by:文章分享

近年来, 越来越多的新烟碱类杀虫剂由于对蜜蜂存在的安全问题陆续被欧盟和美国禁用. 本研究通过虚拟筛选结合生物活性评价的双重策略实现骨架跃迁, 发现苯乙酰腙类化合物VS-04对大豆蚜表现出较好的杀虫活性, 且化合物VS-04和蜜蜂烟碱型乙酰胆碱受体(nAChRs)的作用模式与传统的吡虫啉显著不同. 新合成的苯乙酰腙类似物3c和3e在500 μg/mL浓度下对大豆蚜致死率为70%, 具有中等活性. 更重要的是, 通过蜜蜂毒性研究确证化合物3c和3e对蜜蜂表现出极低的急性接触毒性, 其LD50值(30.35和124.4 μg/bee)比吡虫啉(0.019 μg/bee)低3~4个数量级. 通过化合物3m晶体结构确证化合物3c的酰腙结构以L型低能构象指向蜜蜂受体α亚基, 进一步预示其低蜂毒特征. 基于化合物3e进一步优化得到的化合物4f和4g对大豆蚜表现出更好的致死活性, 其LC50值分别是83.42和66.44 μg/mL, 优于化合物3e (LC50=147.30 μg/mL). 这一研究将有利于以不同昆虫nAChR结构为靶标发现环境友好的新烟碱替代物.
杨朝凯, 武霞, 张晋陆, 路星星, 李想, 蒋志洋, 宋敦伦, 段红霞, 杨新玲. 基于昆虫nAChR选择性的新型低蜂毒苯乙酰腙类似物的筛选和优化[J]. 有机化学, 2021, 41(7): 2774-2787.
Zhaokai Yang, Xia Wu, Jinlu Zhang, Xingxing Lu, Xiang Li, Zhiyang Jiang, Dunlun Song, Hongxia Duan, Xinling Yang. Screening and Optimization of Novel Low Bee-Toxicity Phenylace- tohydrazone Compounds Based on Insect nAChR Selectivity[J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2774-2787.



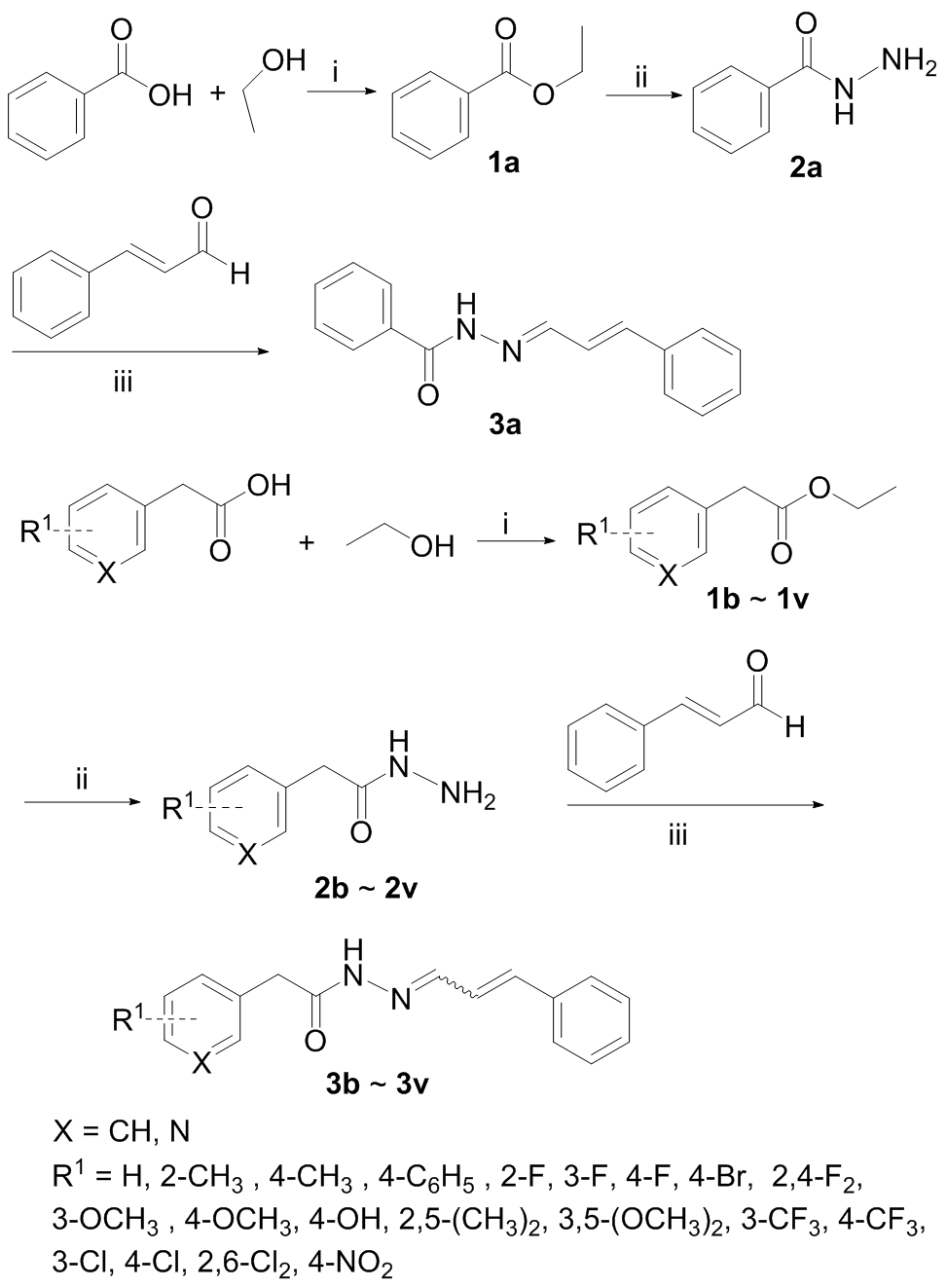
| No. | X | R | n | (Mortality rate±St. Da)/% |
|---|---|---|---|---|
| 3a | CH | H | 0 | 32.32±3.16 |
| 3b | CH | H | 1 | 65.40±3.67 |
| 3c | CH | 4-CH3 | 1 | 71.86±2.93 |
| 3d | CH | 4-C6H5 | 1 | 73.44±2.19 |
| 3e | CH | 4-F | 1 | 70.16±1.22 |
| 3f | CH | 2-F | 1 | 70.72±1.88 |
| 3g | CH | 2-CH3 | 1 | 53.77±3.05 |
| 3h | CH | 3-F | 1 | 43.69±3.48 |
| 3i | CH | 3-OCH3 | 1 | 41.74±4.34 |
| 3j | CH | 4-CF3 | 1 | 28.52±2.12 |
| 3k | CH | 4-OCH3 | 1 | 36.09±1.93 |
| 3l | CH | 4-OH | 1 | 54.75±4.60 |
| 3m | CH | 4-Br | 1 | 29.39±5.92 |
| 3n | CH | 2,5-(CH3)2 | 1 | 27.38±6.35 |
| 3o | CH | 3,5-(OCH3)2 | 1 | 19.89±3.59 |
| 3p | CH | 3-CF3 | 1 | 16.05±1.34 |
| 3q | CH | 2,6-Cl2 | 1 | 15.93±3.06 |
| 3r | CH | 3-Cl | 1 | 25.86±1.91 |
| 3s | CH | 4-NO2 | 1 | 15.61±1.47 |
| 3t | CH | 4-Cl | 1 | 11.01±3.52 |
| 3u | CH | 2,4-F | 1 | 60.22±1.06 |
| 3v | N | H | 1 | 65.65±0.90 |
| Imidacloprid | 92.40±2.34 |
| No. | X | R | n | (Mortality rate±St. Da)/% |
|---|---|---|---|---|
| 3a | CH | H | 0 | 32.32±3.16 |
| 3b | CH | H | 1 | 65.40±3.67 |
| 3c | CH | 4-CH3 | 1 | 71.86±2.93 |
| 3d | CH | 4-C6H5 | 1 | 73.44±2.19 |
| 3e | CH | 4-F | 1 | 70.16±1.22 |
| 3f | CH | 2-F | 1 | 70.72±1.88 |
| 3g | CH | 2-CH3 | 1 | 53.77±3.05 |
| 3h | CH | 3-F | 1 | 43.69±3.48 |
| 3i | CH | 3-OCH3 | 1 | 41.74±4.34 |
| 3j | CH | 4-CF3 | 1 | 28.52±2.12 |
| 3k | CH | 4-OCH3 | 1 | 36.09±1.93 |
| 3l | CH | 4-OH | 1 | 54.75±4.60 |
| 3m | CH | 4-Br | 1 | 29.39±5.92 |
| 3n | CH | 2,5-(CH3)2 | 1 | 27.38±6.35 |
| 3o | CH | 3,5-(OCH3)2 | 1 | 19.89±3.59 |
| 3p | CH | 3-CF3 | 1 | 16.05±1.34 |
| 3q | CH | 2,6-Cl2 | 1 | 15.93±3.06 |
| 3r | CH | 3-Cl | 1 | 25.86±1.91 |
| 3s | CH | 4-NO2 | 1 | 15.61±1.47 |
| 3t | CH | 4-Cl | 1 | 11.01±3.52 |
| 3u | CH | 2,4-F | 1 | 60.22±1.06 |
| 3v | N | H | 1 | 65.65±0.90 |
| Imidacloprid | 92.40±2.34 |
| No. | LD50/(μg•bee–1) | Equation | R2 | 95% CIa |
|---|---|---|---|---|
| 3c | 30.35 | y=–1.829+1.234lgx | 0.944 | 23.075~44.696 |
| 3e | 124.4 | y=–2.458+1.174lgx | 0.937 | 86.668~220.656 |
| Imidacloprid | 0.019/0.081[ | y=3.582+2.076lgx | 0.992 | 0.011~0.028 |
| No. | LD50/(μg•bee–1) | Equation | R2 | 95% CIa |
|---|---|---|---|---|
| 3c | 30.35 | y=–1.829+1.234lgx | 0.944 | 23.075~44.696 |
| 3e | 124.4 | y=–2.458+1.174lgx | 0.937 | 86.668~220.656 |
| Imidacloprid | 0.019/0.081[ | y=3.582+2.076lgx | 0.992 | 0.011~0.028 |
| No. | R | X | (Mortality rate±St. Da)/% |
|---|---|---|---|
| 4a | 4-OCH3 | CH | 29.37±1.61 |
| 4b | 2,6-Cl | CH | 53.73±8.50 |
| 4c | 3-Br | CH | 51.95±0.00 |
| 4d | 4-NO2 | CH | 50.17±4.71 |
| 4e | 4-CH(CH3)2 | CH | 24.44±7.66 |
| 4f | 4-Cl | CH | 70.25±2.10 |
| 4g | H | N | 82.63±5.70 |
| 4h | 4-CF3 | CH | 42.65±1.31 |
| 4i | 4-Br | CH | 43.98±6.12 |
| 4j | 3-Cl | CH | 66.53±10.64 |
| 4k | 3-F | CH | 46.81±4.31 |
| 4l | 4-CH3 | CH | 54.12±4.50 |
| 4m | 4-F | CH | 57.70±8.88 |
| 4n | H | CH | 20.85±2.92 |
| 4o | 2-Cl | CH | 75.08±6.23 |
| Imidacloprid | 92.88±2.36 |
| No. | R | X | (Mortality rate±St. Da)/% |
|---|---|---|---|
| 4a | 4-OCH3 | CH | 29.37±1.61 |
| 4b | 2,6-Cl | CH | 53.73±8.50 |
| 4c | 3-Br | CH | 51.95±0.00 |
| 4d | 4-NO2 | CH | 50.17±4.71 |
| 4e | 4-CH(CH3)2 | CH | 24.44±7.66 |
| 4f | 4-Cl | CH | 70.25±2.10 |
| 4g | H | N | 82.63±5.70 |
| 4h | 4-CF3 | CH | 42.65±1.31 |
| 4i | 4-Br | CH | 43.98±6.12 |
| 4j | 3-Cl | CH | 66.53±10.64 |
| 4k | 3-F | CH | 46.81±4.31 |
| 4l | 4-CH3 | CH | 54.12±4.50 |
| 4m | 4-F | CH | 57.70±8.88 |
| 4n | H | CH | 20.85±2.92 |
| 4o | 2-Cl | CH | 75.08±6.23 |
| Imidacloprid | 92.88±2.36 |
| No. | LC50/(μg•mL–1) | Equation | R2 | 95% CIa |
|---|---|---|---|---|
| 3e | 147.30 | y=–2.904+1.339lgx | 0.988 | 110.82~208.096 |
| 4f | 83.42 | y=–2.626+1.367lgx | 0.938 | 36.851~229.430 |
| 4g | 66.44 | y=–2.443+1.340lgx | 0.985 | 50.397~88.129 |
| Imidacloprid | 29.87 | y=–2.406+1.631lgx | 0.930 | 13.787~54.372 |
| No. | LC50/(μg•mL–1) | Equation | R2 | 95% CIa |
|---|---|---|---|---|
| 3e | 147.30 | y=–2.904+1.339lgx | 0.988 | 110.82~208.096 |
| 4f | 83.42 | y=–2.626+1.367lgx | 0.938 | 36.851~229.430 |
| 4g | 66.44 | y=–2.443+1.340lgx | 0.985 | 50.397~88.129 |
| Imidacloprid | 29.87 | y=–2.406+1.631lgx | 0.930 | 13.787~54.372 |
| [1] |
Dedryver,C. A.; Le Ralec, A.; Fabre, F. C. R. Biol. 2010, 333,539.
doi: 10.1016/j.crvi.2010.03.009 |
| [2] |
Bonnemain,J. L. C. R. Biol. 2010, 333,461.
doi: 10.1016/j.crvi.2010.04.002 |
| [3] |
Chappell,M. R.; Braman,S. K.; Williams-Woodward, J.; Knox, G. J. Environ. Hort. 2012, 30,161.
|
| [4] |
Mauck,K. E.; De Moraes,C. M.; Mescher,M. C. Plant, Cell Environ. 2014, 37,1427.
doi: 10.1111/pce.2014.37.issue-6 |
| [5] |
Voss, G.; Ramos, G. Chemistry of Crop Protection: Progress and Prospects in Science and Regulation, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003.
|
| [6] |
Tomizawa, M.; Casida,J. E. Annu. Rev. Pharmacol. Toxicol. 2005, 45,247.
doi: 10.1146/annurev.pharmtox.45.120403.095930 |
| [7] |
Kagabu, S. J. Agric. Food Chem. 2011, 59,2887.
doi: 10.1021/jf101824y |
| [8] |
Chen, L.; Wang,B. Q.; Zhao,Y. C.; Yan,S. J.; Lin, J. Chin. J. Org. Chem. 2017, 37,1433 (in Chinese).
doi: 10.6023/cjoc201612038 |
|
( 陈亮, 王保取, 赵宇澄, 严胜骄, 林军, 有机化学, 2017, 37,1433.)
|
|
| [9] |
Chen,Y. B.; Fan, J.; Xia, S.; Chen,J. G.; Xu,X. Y.; Li, Z. Chin. J. Org. Chem. 2014, 34,409 (in Chinese).
doi: 10.6023/cjoc201308033 |
|
( 陈寅波, 范杰, 夏爽, 程家高, 徐晓勇, 李忠, 有机化学, 2014, 34,409.)
|
|
| [10] |
Tian,Z. Z.; Wang,X. H.; Li,D. M. Chin. J. Org. Chem. 2020, 40,4332 (in Chinese).
doi: 10.6023/cjoc202006056 |
|
( 田忠贞, 王先浩, 李冬梅, 有机化学, 2020, 40,4332.)
|
|
| [11] |
Kagabu, S.; Nishimura, K.; Naruse, Y.; Ohno, I. J. Pestic. Sci. 2008, 33,58.
doi: 10.1584/jpestics.R07-25 |
| [12] |
Kagabu, S.; Ishihara, R.; Hieda, Y.; Nishimura, K.; Naruse, Y. J. Agric. Food Chem. 2007, 55,812.
doi: 10.1021/jf0623440 |
| [13] |
Tomizawa, M.; Casida,J. E. Acc. Chem. Res. 2009, 42,260.
doi: 10.1021/ar800131p |
| [14] |
Jeschke, P.; Nauen, R; Beck,M. E. Angew. Chem.,Int. Ed. 2013, 52,9464.
doi: 10.1002/anie.v52.36 |
| [15] |
Insecticide Resistance Action Committee: The IRAC mode of action classification. https://irac-online.org/content/uploads/MoA_ Group_4.pdf
|
| [16] |
Suchail, S.; Guez, D.; Belzunces,L. P. Environ. Toxicol. Chem. 2001, 20,2482.
doi: 10.1002/etc.v20:11 |
| [17] |
Yang, Y.; Ma, S.; Liu, F.; Wang, Q.; Wang, X.; Hou,C. S.; Wu,Y. Y.; Gao, J.; Zhang, L.; Liu,Y. J.; Diao,Q. Y.; Dai, P. Pest Manage. Sci. 2019, 76,978.
doi: 10.1002/ps.v76.3 |
| [18] |
Jacob,C. R.O.; Malaquias,J. B.; Zanardi,O. Z.; Silva,C. A.S.; Jacob,J. F.O.; Yamamoto,P. T. Ecotoxicology 2019, 28,744.
doi: 10.1007/s10646-019-02070-w |
| [19] |
Wade, A.; Lin,C. H.; Kurkul, C.; Regan,R. E.; Reed M, Johnson,R. M. Insects 2019, 10,20.
doi: 10.3390/insects10010020 |
| [20] |
Schwartz,K. R.; Minor, H.; Magro, C.; McConnell, J.; Capani, J.; Griffin, J.; Doebel, H. J. Apic. Res. 2020, 60,431.
doi: 10.1080/00218839.2020.1866233 |
| [21] |
Liu, Q.; Zhou,X. X.; Yuan,S. K. Agrochemicals 2020, 59,660 (in Chinese).
|
|
( 刘琼, 周欣欣, 袁善奎, 农药, 2020, 59,660.)
|
|
| [22] |
Shi,J. L.; Yang, L.; Liao,C. H. Agrochemicals, 2019, 58,6 (in Chinese).
|
|
( 史晶亮, 杨乐, 廖春华, 农药, 2019, 58,6.)
|
|
| [23] |
IUPAC Pesticide Properties Database:
|
| [24] |
Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux,J. F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtyes, A. Science 2012, 336,348.
doi: 10.1126/science.1215039 |
| [25] |
Cresswell,J. E.; Page,C. J.; Uygun,M. B.; Holmbergh, M.; Li,Y. R.; Wheeler,J. G.; Laycock, I.; Pook,C. J.; De Ibarra,N. H.; Smirnoff, N.; Tyler,C. R. Zoology 2012, 115,365.
doi: 10.1016/j.zool.2012.05.003 |
| [26] |
Laycock, I.; Lenthall,K. M.; Barratt,A. T.; Cresswell,J. E. Ecotoxicology 2012, 21,1937.
doi: 10.1007/s10646-012-0927-y |
| [27] |
Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Science 2014, 346,1360.
doi: 10.1126/science.1257259 |
| [28] |
Whitehorn,P. R.; O'Connor, S.; Wackers,F. L.; Goulson, D. Science 2012, 336,351.
doi: 10.1126/science.1215025 |
| [29] |
Rundlöf, M.; Andersson,G. K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt,B. K.; Pedersen,T. R.; Yourstone, J.; Smith,H. G. Nature 2015, 521,77.
doi: 10.1038/nature14420 |
| [30] |
Woodcock,B. A.; Bullock,J. M.; Shore,R. F.; Heard,M. S.; Pereira,M. G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; Peyton, J.; Hulmes, S.; Hulmes, L.; Sárospataki, M.; Saure, C.; Edwards, M.; Genersch, E.; Knäbe, S.; Pywell,R. F. Science 2017, 356,1393.
doi: 10.1126/science.aaa1190 |
| [31] |
Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel,H. S.; Malena,D. A.; Gajiwala,P. H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Science 2017, 356,1395.
doi: 10.1126/science.aam7470 |
| [32] |
Stokstad, E. Science 2017, 356,1321.
doi: 10.1126/science.356.6345.1321 |
| [33] |
Butler, D. Nature 2018,DOI: 10.1038/d41586-018-04987-4.
doi: 10.1038/d41586-018-04987-4 |
| [34] |
Erik, S. Science 2018,DOI: 10.1126/science.aau0152.
doi: 10.1126/science.aau0152 |
| [35] |
Hou, J.; Xie, W.; Hong, D.; Zhang,W. H.; Li, F.; Qian, Y.; Han, C. Food Chem. 2019, 270,204.
doi: 10.1016/j.foodchem.2018.07.068 |
| [36] |
Nauen, R.; Jeschke, P.; Velten, R.; Beck,M. E.; Ebbinghaus- Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Pest Manage. Sci. 2015, 71,850.
doi: 10.1002/ps.2015.71.issue-6 |
| [37] |
Jeschke, P.; Nauen, R.; Gutbrod, O.; Beck,M. E.; Matthiesen, S.; Haas, M.; Velten, R. Pestic. Biochem. Phys. 2014, 121,31.
doi: 10.1016/j.pestbp.2014.10.011 |
| [38] |
Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin,K. A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shikako, R.; Furutani, S.; Ihara, M.; Matsuda, K.; Mitomi, M.; Kagabu, S.; Uomoto, K.; Tomizawa, M. J. Agric. Food Chem. 2017, 65,7865.
doi: 10.1021/acs.jafc.7b02924 |
| [39] |
Hesselbach, H.; Scheiner, R. Sci. Rep. 2018, 8,4954.
doi: 10.1038/s41598-018-23200-0 |
| [40] |
Hu, Y.; Stumpfe, D.; Bajorath, J. J. Med. Chem. 2017, 60,1238.
doi: 10.1021/acs.jmedchem.6b01437 |
| [41] |
Lamberth, C. Pest Magnage. Sci. 2018, 74,282.
|
| [42] |
Chen,Z. L.; Yao,X. M.; Dong,F. S.; Duan,H. X.; Shao,X. S.; Chen, X.; Yang, T.; Wang,G. R.; Zheng,Y. Q. Environ. Int. 2019, 130,104854.
doi: 10.1016/j.envint.2019.05.048 |
| [43] |
Smart,B. E. J. Fluorine. Chem. 2001, 109,3.
|
| [44] |
Jeschke, P. Pest Manage. Sci. 2016, 72,433.
doi: 10.1002/ps.2016.72.issue-3 |
| [45] |
Badawy,M. E.I.; Nasr,H. M.; Rabea,E. I. Apidologie 2015, 46, 177.
|
| [46] |
Talley,T. T.; Harel, M.; Hibbs,R. E.; Radic, Z.; Tomizawa, M.; Casida,J. E.; Taylor, P. Proc. Natl. Acad. Sci. U. S. A. 2008, 105,7606.
doi: 10.1073/pnas.0802197105 |
| [47] |
Chandrasena, D.; Difonzo, C.; Byrne, A. J. Econ. Entomol. 2011, 104,1357.
doi: 10.1603/EC10414 |
| [48] |
Magalhaes,L. C.; Hunt, T.E; Siegfried,B. D. Entomol. Exp. Appl. 2008, 128,330.
doi: 10.1111/eea.2008.128.issue-2 |
| [49] |
Screening Compounds and Libraries for Hit identification: https://www.maybridge.com
|
| [50] |
Lipinski,C. A.; Lombardo, F.; Dominy,B. W.; Feeney,P. J. Adv. Drug Delivery Rev. 2012, 64,4.
doi: 10.1016/j.addr.2012.09.019 |
| [51] |
Open Source Independent Review and Interpretation System: . https://www.ncbi.nlm.nih.gov/osiris/download
|
| [52] |
Clark,R. D.; Strizhev, A.; Leonard,J. M.; Blake,J. F.; Matthew,J. B. J. Mol. Graphics Modell. 2002, 20, 281.
|
| [53] |
v. Auwers K.; Daniel W. J. Prakt. Chem. (Leipzig) 1925, 110,235.
|
| [54] |
RAC Approved Test Methods for resistance detection in the world's major insect pests:. https://www.irac-online.org/methods/ aphids-adultnymphs
|
| [55] |
Hanson,A. A.; Menger-Anderson, J.; Silverstein, C.; Potter,B. D.; MacRae,I. V.; Hodgson,E. W.; Koch,R. L. J. Econ. Entomol. 2017, 110,2235.
doi: 10.1093/jee/tox235 |
| [56] |
Bliss,C. I. Science 1934, 79,38.
doi: 10.1126/science.79.2037.38 |
| [1] | 李想, 朱凯, 韩清, 路星星, 李明君, 凌云, 段红霞. 新型呋喃α-丁烯内酯类化合物的设计、合成及其生物活性研究[J]. 有机化学, 2023, 43(1): 202-213. |
| [2] | 田忠贞, 王先浩, 李冬梅. 新型含二硫环戊烯酮结构新烟碱类衍生物的合成与生物活性[J]. 有机化学, 2020, 40(12): 4332-4338. |
| [3] | 陈亮, 王保取, 赵宇澄, 严胜骄, 林军. 一锅法合成多取代色酮并双环吡啶类化合物[J]. 有机化学, 2017, 37(6): 1433-1442. |
| [4] | 陈寅波, 范杰, 夏爽, 程家高, 徐晓勇, 李忠. 新型双环新烟碱类似物的合成及其生物活性[J]. 有机化学, 2014, 34(2): 409-413. |
| [5] | 薛思佳, 马旭波, 步洪飞. 1,5-二取代六氢三嗪-2-N-硝基亚胺的合成和杀虫活性的测定[J]. 有机化学, 2011, 31(06): 881-885. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||