有机化学 ›› 2022, Vol. 42 ›› Issue (2): 631-640.DOI: 10.6023/cjoc202108050 上一篇 下一篇
研究论文
孙亚敏a, 李锡勇a, 袁金伟b,*( ), 余加琳b, 刘帅楠b
), 余加琳b, 刘帅楠b
收稿日期:2021-08-26
修回日期:2021-09-26
发布日期:2022-02-24
通讯作者:
袁金伟
基金资助:
Yamin Suna, Xiyong Lia, Jinwei Yuanb( ), Jialin Yub, Shuainan Liub
), Jialin Yub, Shuainan Liub
Received:2021-08-26
Revised:2021-09-26
Published:2022-02-24
Contact:
Jinwei Yuan
Supported by:文章分享

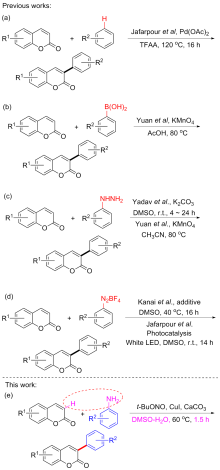
以香豆素和芳香胺为原料, 在温和条件下通过CuI催化合成3-芳基香豆素衍生物. 该合成方法简单地利用易得的原料为底物, 廉价的金属作为催化剂, 提供具有良好官能团耐受性的3-芳基香豆素, 产率适中或较高. 此外, 控制实验表明该反应是通过自由基途径进行的.
孙亚敏, 李锡勇, 袁金伟, 余加琳, 刘帅楠. 温和条件下以芳基胺为原料CuI催化下区域选择性合成3-芳基香豆素[J]. 有机化学, 2022, 42(2): 631-640.
Yamin Sun, Xiyong Li, Jinwei Yuan, Jialin Yu, Shuainan Liu. CuI-Catalyzed Regioselective Synthesis of 3-Arylcoumarins with Arylamines under Mild Conditions[J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 631-640.
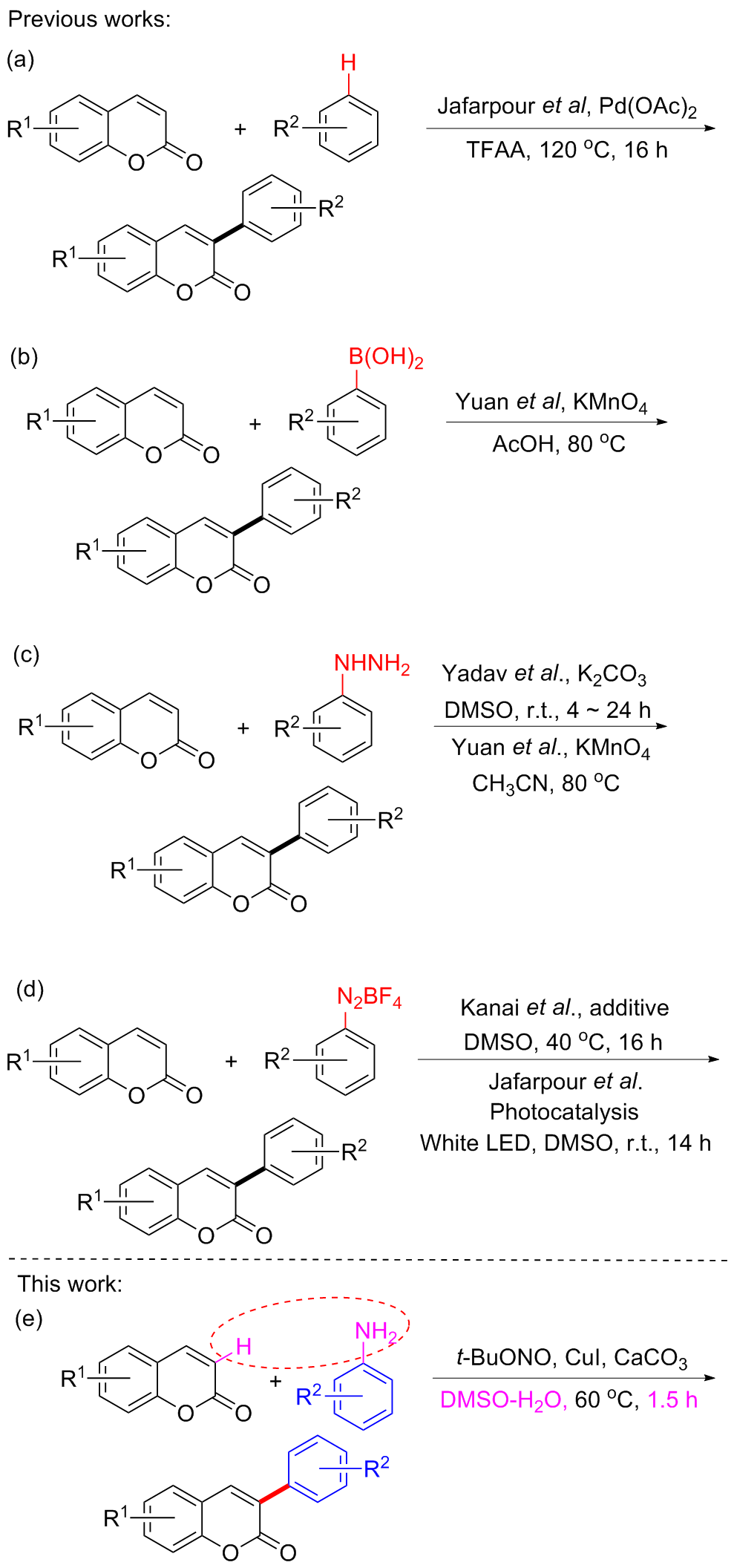
| Entry | Catalyst (equiv.) | Base (equiv.) | Solvent | Temp/℃ | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Cu(OAc)2 (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 31 |
| 2 | CuSO4 (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 41 |
| 3 | CuO (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 32 |
| 4 | CuI2 (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 10 |
| 5 | CuCl (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 38 |
| 6 | CuBr (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 40 |
| 7 | CuI (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 48 |
| 8 | CuI (0.05) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 22 |
| 9 | CuI (0.15) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 53 |
| 10 | CuI (0.2) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 47 |
| 11 | CuI (0.15) | Cs2CO3 (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 40 |
| 12 | CuI (0.15) | K2CO3 (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 48 |
| 13 | CuI (0.15) | CaCO3 (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 60 |
| 14 | CuI (0.15) | NaOAc (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 37 |
| 15 | CuI (0.15) | CaO (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 50 |
| 16 | CuI (0.15) | CaCO3 (1.0) | H2O | 25 | 2.0 | Trace |
| 17 | CuI (0.15) | CaCO3 (1.0) | CH3CN | 25 | 2.0 | <5 |
| 18 | CuI (0.15) | CaCO3 (1.0) | DMSO | 25 | 2.0 | <5 |
| 19 | CuI (0.15) | CaCO3 (1.0) | CH2Cl2 | 25 | 2.0 | 42 |
| 20 | CuI (0.15) | CaCO3 (1.0) | CH2Cl2/H2O (V∶V=1∶1) | 25 | 2.0 | 15 |
| 21 | CuI (0.15) | CaCO3 (1.0) | CH3CN/H2O (V∶V=1∶1) | 25 | 2.0 | 10 |
| 22 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=1∶1) | 25 | 2.0 | 48 |
| 23 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=2∶1) | 25 | 2.0 | 55 |
| 24 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 25 | 2.0 | 67 |
| 25 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 40 | 2.0 | 52 |
| 26 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 50 | 2.0 | 61 |
| 27 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 2.0 | 75 |
| 28 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 70 | 2.0 | 70 |
| 29 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 0.5 | 46 |
| 30 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 1.0 | 55 |
| 31 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 1.5 | 77 |
| 32 | — | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 25 | 2.0 | 23 |
| Entry | Catalyst (equiv.) | Base (equiv.) | Solvent | Temp/℃ | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Cu(OAc)2 (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 31 |
| 2 | CuSO4 (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 41 |
| 3 | CuO (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 32 |
| 4 | CuI2 (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 10 |
| 5 | CuCl (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 38 |
| 6 | CuBr (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 40 |
| 7 | CuI (0.1) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 48 |
| 8 | CuI (0.05) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 22 |
| 9 | CuI (0.15) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 53 |
| 10 | CuI (0.2) | — | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 47 |
| 11 | CuI (0.15) | Cs2CO3 (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 40 |
| 12 | CuI (0.15) | K2CO3 (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 48 |
| 13 | CuI (0.15) | CaCO3 (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 60 |
| 14 | CuI (0.15) | NaOAc (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 37 |
| 15 | CuI (0.15) | CaO (1.0) | Acetone/H2O (V∶V=2∶1) | 25 | 2.0 | 50 |
| 16 | CuI (0.15) | CaCO3 (1.0) | H2O | 25 | 2.0 | Trace |
| 17 | CuI (0.15) | CaCO3 (1.0) | CH3CN | 25 | 2.0 | <5 |
| 18 | CuI (0.15) | CaCO3 (1.0) | DMSO | 25 | 2.0 | <5 |
| 19 | CuI (0.15) | CaCO3 (1.0) | CH2Cl2 | 25 | 2.0 | 42 |
| 20 | CuI (0.15) | CaCO3 (1.0) | CH2Cl2/H2O (V∶V=1∶1) | 25 | 2.0 | 15 |
| 21 | CuI (0.15) | CaCO3 (1.0) | CH3CN/H2O (V∶V=1∶1) | 25 | 2.0 | 10 |
| 22 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=1∶1) | 25 | 2.0 | 48 |
| 23 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=2∶1) | 25 | 2.0 | 55 |
| 24 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 25 | 2.0 | 67 |
| 25 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 40 | 2.0 | 52 |
| 26 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 50 | 2.0 | 61 |
| 27 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 2.0 | 75 |
| 28 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 70 | 2.0 | 70 |
| 29 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 0.5 | 46 |
| 30 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 1.0 | 55 |
| 31 | CuI (0.15) | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 60 | 1.5 | 77 |
| 32 | — | CaCO3 (1.0) | DMSO/H2O (V∶V=3∶1) | 25 | 2.0 | 23 |
| [1] |
(a) Kostova, I. Curr. Med. Chem.: Anti-Cancer Agents 2005, 5, 29.
|
|
(b) Hoult, J. R. S.; Paya, M. Gen. Pharmacol. 1996, 27, 713.
doi: 10.1016/0306-3623(95)02112-4 |
|
|
(c) Kabeya, L. M.; deMarchi, A. A.; Kanashiro, A.; Lopes, N. P.; da Silva, C. H. T. P.; Pupo, M. T.; Lucisano-Valim, Y. M. Bioorg. Med. Chem. 2007, 15, 1516.
doi: 10.1016/j.bmc.2006.10.068 |
|
| [2] |
(a) Dayam, R.; Gundla, R.; Al-Mawsawi, L. Q.; Neamati, N. Med. Res. Rev. 2008, 28, 118.
doi: 10.1002/(ISSN)1098-1128 |
|
(b) Thuong, P. T.; Hung, T. M.; Ngoc, T. M.; Ha, D. T.; Min, B. S.; Kwack, S. J.; Kang, T. S.; Bae, K. Phytother. Res. 2010, 24, 101.
doi: 10.1002/ptr.v24:1 |
|
|
(c) Koefod, R. S.; Mann, K. R. Inorg. Chem. 1989, 28, 2285.
doi: 10.1021/ic00311a009 |
|
| [3] |
(a) de Souza Santos, M.; de Morais Del Lama, M. P. F.; Deliberto, L. A.; da Silva Emery, F.; Pupo, M. T.; Naal, R. M. Z. G. Arch. Pharmacal Res. 2013, 36, 731.
doi: 10.1007/s12272-013-0084-8 |
|
(b) Zhao, H.; Yan, B. L.; Peterson, B.; Luo, J.; Xu, T.; Du, W.; Xu, Q.; Tu, Z.; Brekken, R. A.; Ren, X.; Bullock, A. N.; Liang, G.; Lu, X.; Oracid, K. D. ACS Med. Chem. Lett. 2012, 3, 327.
doi: 10.1021/ml300018e |
|
|
(c) Wang, C.; Wu, C.; Zhu, J.; Miller, R. H.; Wang, Y. J. Med. Chem. 2011, 54, 2331.
doi: 10.1021/jm101489w |
|
|
(d) Reddie, K. G.; Humphries, W. H.; Bain, C. P.; Payne, C. K.; Kemp, M. L.; Murthy, N. Org. Lett. 2012, 14, 680.
doi: 10.1021/ol203105c |
|
| [4] |
Jafarpour, F.; Abbasnia, M. J. Org. Chem. 2016, 81, 11982.
pmid: 27800677 |
| [5] |
(a) Wang, C.; Mi, X.; Li, Q.; Li, Y.; Huang, M.; Zhang, J.; Wu, Y.; Wu, Y. Tetrahedron 2015, 71, 6689.
doi: 10.1016/j.tet.2015.07.052 |
|
(b) Banerjee, A.; Santra, S. K.; Khatun, N.; Ali, W.; Patel, B. K. Chem. Commun. 2015, 51, 15422.
doi: 10.1039/C5CC06200D |
|
|
(c) Niu, B.; Zhao, W.; Ding, Y.; Bian, Z.; Pittman, C. U.; Zhou, A.; Ge, H. J. Org. Chem. 2015, 80, 7251.
doi: 10.1021/acs.joc.5b00800 |
|
|
(d) Liu, L.; Pan, N.; Sheng, W.; Su, L.; Liu, L.; Dong, J.; Zhou, Y. B.; Yin, S. F. Adv. Synth. Catal. 2019, 361, 4126.
doi: 10.1002/adsc.v361.17 |
|
|
(e) Trinh, K. H.; Tran, P. H.; Nguyen, T. T.; Doan, S. H.; Le, M. V.; Nguyen, T. T.; Phan, N. T. S. Appl. Organomet. Chem. 2020, 34, e5515.
|
|
| [6] |
(a) Kim, K.; Kim, Y.; Hong, S. Chem. Commun. 2013, 49, 196.
doi: 10.1039/C2CC37676H |
|
(b) Min, M.; Hong, S. Chem. Commun. 2012, 48, 9613.
doi: 10.1039/c2cc34551j |
|
| [7] |
(a) He, C. Y.; Kong, J.; Li, X.; Li, X.; Yao, Q.; Yuan, F. M. J. Org. Chem. 2017, 82, 910.
doi: 10.1021/acs.joc.6b02316 |
|
(b) Huang, C. M.; Li, J.; Wang, S. L.; Ai, J. J.; Liu, X. Y.; Rao, W. D.; Wang, S. Y. J. Org. Chem. 2021, 86, 8437.
doi: 10.1021/acs.joc.1c00965 |
|
|
(c) Zhu, X. L.; Huang, Y.; Xu, X. H.; Qing, F. L. Org. Lett. 2020, 22, 5451.
doi: 10.1021/acs.orglett.0c01826 |
|
| [8] |
(a) Mi, X.; Huang, M.; Zhang, J.; Wang, C.; Wu, Y. Org. Lett. 2013, 15, 6266;
doi: 10.1021/ol4031167 |
|
(b) Kim, I.; Min, M.; Kang, D.; Kim, K.; Hong, S. Org. Lett. 2017, 19, 1394.
doi: 10.1021/acs.orglett.7b00299 |
|
|
(c) Li, Q. R.; Zhao, X. L.; Li, Y. B.; Huang, M. M.; Kim. J. K.; Wu, Y. J. Org. Biomol. Chem., 2017, 15, 9775.
doi: 10.1039/C7OB02478A |
|
|
(d) Yuan, J. W.; Li, Y. Z.; Yang, L. R.; Mai, W. P.; Mao, P.; Xiao, Y. M.; Qu, L. B. Tetrahedron 2015, 71, 8178.
doi: 10.1016/j.tet.2015.08.026 |
|
| [9] |
(a) Song, Z.; Ding, C.; Wang, S.; Dai, Q.; Sheng, Y.; Zheng, Z.; Liang, G. Chem. Commun. 2020, 56, 1847.
doi: 10.1039/C9CC09001K |
|
(b) Mostardeiro, V. B.; Dilelio, M. C.; Kaufman, T. S.; Silveira, C. C. RSC Adv. 2020, 10, 482.
doi: 10.1039/C9RA09545D |
|
| [10] |
(a) Natarajan, P.; Chuskit, D. Green Chem. 2021, 23, 4873.
doi: 10.1039/D1GC01382C |
|
(b) Chen, Z.; Bai, X.; Sun, J.; Xu, Y. J. Org. Chem. 2020, 85, 7674.
doi: 10.1021/acs.joc.0c00113 |
|
|
(c) Zhou, S. L.; Guo, L. N.; Duan, X. H. Eur. J. Org. Chem. 2014, 2014, 8094.
doi: 10.1002/ejoc.v2014.36 |
|
| [11] |
(a) Kang, D.; Ahn, K.; Hong, S. Asian J. Org. Chem. 2018, 7, 1136.
doi: 10.1002/ajoc.v7.7 |
|
(b) Liu, S. N.; Yuan, J. W.; Qu, L. B. Chin. J. Org. Chem. 2018, 38, 316. (in Chinese)
doi: 10.6023/cjoc201708058 |
|
|
( 刘帅男, 袁金伟, 屈凌波, 有机化学, 2018, 38, 316.)
doi: 10.6023/cjoc201708058 |
|
| [12] |
(a) Gao, P.; Cheng, Y. B.; Yang, F.; Guo, L. N.; Duan, X. H. Tetrahedron Lett. 2019, 60, 150967.
doi: 10.1016/j.tetlet.2019.150967 |
|
(b) Chen, X.; Li, L.; Pei, C.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. J. Org. Chem. 2021, 86, 2772.
doi: 10.1021/acs.joc.0c02739 |
|
| [13] |
(a) You, L.; An, R.; Wang, X.; Li, Y. Bioorg. Med. Chem. Lett. 2010, 20, 7426.
doi: 10.1016/j.bmcl.2010.10.027 |
|
(b) El-Seedi, H. R. J. Nat. Prod. 2007, 17, 118.
|
|
|
(c) Narvaez-Mastache, J.; Novillo, M. F.; Delgado, G. Phytochemistry 2008, 69, 451.
doi: 10.1016/j.phytochem.2007.07.019 |
|
| [14] |
(a) Matos, M. J.; Viña, D.; Picciau, C.; Orallo, F.; Santana, L.; Uriarte, E. Bioorg. Med. Chem. Lett. 2009, 19, 5053.
|
|
(b) Matos, M. J.; Viña, D.; Quezada, E.; Picciau, C. Delogu, G.; Orallo, F.; Santana, L.; Uriarte, E. Bioorg. Med. Chem. Lett. 2009, 19, 3268.
doi: 10.1016/j.bmcl.2009.04.085 |
|
|
(c) Matos, M. J.; Terán, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. J. Med. Chem. 2011, 54, 7127.
doi: 10.1021/jm200716y |
|
| [15] |
Kabeya, L. M.; Marchi, A. A.; Kanashiro, A.; Lopes, N. P.; da Silva, C. H. T. P.; Pupo, M. T.; Lucisano-Valim, Y. M. Bioorg. Med. Chem. 2007, 15, 1515.
doi: 10.1016/j.bmcl.2004.12.061 |
| [16] |
(a) Olmedo, D.; Sancho, R.; Bedoya, L. M.; Lopez-Perez, J. L.; Olmo, E. D.; Munoz, E.; Alcami, J.; Gupta, M. P.; Feliciano, A. S. Molecules 2012, 17, 9245.
doi: 10.3390/molecules17089245 |
|
(b) Ong, E. B. B.; Watanabe, N.; Saito, A.; Futamura, Y.; Galil, K. H. A. E.; Koito, A.; Najimudin, N.; Osada, H. J. Biol. Chem. 2011, 286, 14049.
doi: 10.1074/jbc.M110.185397 |
|
| [17] |
(a) Schiedel, M. S.; Briehn, C. A.; Bäuerle P. Angew. Chem., nt. Ed. 2001, 40, 4677.
doi: 10.1002/1521-3773(20011217)40:24【-逻*辑*与-】#x00026;lt;【-逻*辑*与-】#x00026;gt;1.0.CO;2-C pmid: 11055341 |
|
(b) Edwards, P. D.; Mauger, R. C.; Cottrell, K. M.; Morris, F. X.; Pine, K. K.; Sylvester, M. A.; Scott, C. W.; Furlong, S. T. Bioorg. Med. Chem. Lett. 2000, 10, 2291.
pmid: 11055341 |
|
|
(c) Lee, M. T.; Yen, C. K.; Yang, W. P.; Chen, H. H.; Liao, C. H.; Tsai, C. H.; Chen, C. H. Org. Lett. 2004, 6, 1241.
doi: 10.1021/ol049903d pmid: 11055341 |
|
|
(d) Swanson, S. A.; Wallraff, G. M.; Chen, J. P.; Zhang, W. J.; Bozano, L. D.; Carter, K. R.; Salem, J. R.; Villa, R.; Scott, J. C. Chem. Mater. 2003, 15, 2305.
doi: 10.1021/cm021056q pmid: 11055341 |
|
|
(e) Chang, C. H.; Cheng, H. C.; Lu, Y. J.; Tien, K. C.; Lin, H. W.; Lin, C. L.; Yang, C. J.; Wu, C. C. Org. Electron. 2010, 11, 247.
doi: 10.1016/j.orgel.2009.11.002 pmid: 11055341 |
|
| [18] |
(a) Liu, J. M.; Zhang, X.; Shi, L. J.; Liu, M. W.; Yue, Y. Y.; Li, F. W.; Zhao, K. L. Chem. Commun. 2014, 50, 9887.
doi: 10.1039/C4CC04377D |
|
(b) Zeng, H. Y.; Li, C. J. Angew. Chem., nt. Ed., 2014, 53, 13862.
doi: 10.1002/anie.201407589 |
|
|
(c) Sashidhara, K. V.; Palnati, G. R.; Avula, S. R.; Kumar, A. Synlett 2012, 611.
|
|
| [19] |
(a) Unsinn, A.; Wunderlich, S. H.; Knochel, P. Adv. Synth. Catal. 2013, 355, 989.
doi: 10.1002/adsc.201300049 pmid: 22381127 |
|
(b) Messaoudi, S.; Brion, J. D.; Alami, M. Org. Lett. 2012, 14, 1496.
doi: 10.1021/ol300235k pmid: 22381127 |
|
|
(c) Mosrin, M.; Monzon, G.; Bresser, T.; Knochel, P. Chem. Commun. 2009, 37, 5615.
pmid: 22381127 |
|
|
(d) Matos, M. J.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E. Tetrahedron Lett. 2011, 52, 1225.
doi: 10.1016/j.tetlet.2011.01.048 pmid: 22381127 |
|
| [20] |
(a) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 16, 11212.
|
|
(b) Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., nt. Ed. 2012, 51, 10236.
doi: 10.1002/anie.201203269 |
|
|
(c) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
doi: 10.1021/cr100280d |
|
|
(d) Wang, L. L.; Bao, P. L.; Liu, W. W.; Liu, S. T.; Hu, C. S.; Yue, H. L.; Yang, D. S.; Wei, W. Chin. J. Org. Chem. 2018, 38, 3189. (in Chinese)
doi: 10.6023/cjoc201807014 |
|
|
( 王雷雷, 鲍鹏丽, 刘维伟, 刘思彤, 胡昌松, 岳会兰, 杨道山, 魏伟, 有机化学, 2018, 38, 3189.)
doi: 10.6023/cjoc201807014 |
|
|
(e) Dong, D. Q.; Li, G. H.; Chen, D. M.; Sun, Y. Y.; Han, Q. Q.; Wang, Z. L.; Xu, X. M.; Yu, X. Y. Chin. J. Org. Chem. 2020, 40, 1766. (in Chinese)
doi: 10.6023/cjoc202002002 |
|
|
( 董道青, 李光辉, 陈德茂, 孙媛媛, 韩晴晴, 王祖利, 徐鑫明, 于贤勇, 有机化学, 2020, 40, 1766.)
doi: 10.6023/cjoc202002002 |
|
|
(f) Wu, Y.; Chen, J. Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W. M. Green Chem. 2021, 23, 3950.
doi: 10.1039/D1GC00562F |
|
|
(g) Chen, J. Y.; Wu, H. Y.; Gui, Q. W.; Yan, S. S.; Deng, J.; Lin, Y. W.; Cao, Z.; He, W. M. Chin. J. Catal. 2021, 42, 1445.
|
|
| [21] |
Jafarpour, F.; Hazrati, H.; Mohasselyazdi, N.; Khoobi, M.; Shafiee, A. Chem. Commun. 2013, 49, 10935.
doi: 10.1039/c3cc46959j |
| [22] |
She, Z.; Shi, Y.; Huang, Y.; Cheng, Y.; Song, F.; You, J. Chem. Commun. 2014, 50, 13914.
doi: 10.1039/C4CC05827E |
| [23] |
Yuan, Y. W.; Yang, L. R.; Yin, Q. Y.; Mao, P.; Qu, L. B. RSC Adv. 2016, 6, 35936.
doi: 10.1039/C6RA04787D |
| [24] |
(a) Hofmann, J.; Jasch, H.; Heinrich, M. R. J. Org. Chem. 2014, 79, 2314.
doi: 10.1021/jo500063r pmid: 24524356 |
|
(b) Li, M. Y.; Ye, Y. ChemCatChem 2015, 7, 4137.
doi: 10.1002/cctc.201500575 pmid: 24524356 |
|
|
(c) Chen, Z. X.; Wang, G. W. J. Org. Chem. 2005, 70, 2380.
doi: 10.1021/jo047894g pmid: 24524356 |
|
| [25] |
Chauhan, P.; Ravi, M.; Singh, S.; Prajapati, P.; Yadav, P. P. RSC Adv. 2016, 6, 109.
doi: 10.1039/C5RA20954D |
| [26] |
Yuan, J. W.; Li, W. J.; Yang, L. R.; Mao, P.; Xiao, Y. M. Z. Naturforsch. 2016, 71, 1115.
|
| [27] |
Kojima, M.; Oisaki, K.; Kanai, M. Chem. Commun. 2015, 51, 9718.
doi: 10.1039/C5CC02349A |
| [28] |
Moazzam, A.; Jafarpour, F. New J. Chem. 2020, 44, 16692.
doi: 10.1039/D0NJ02012E |
| [29] |
(a) Jafarpour, F.; Olia, M. B. A.; Hazrati, H. Adv. Synth. Catal. 2013, 355, 3407
doi: 10.1002/adsc.201300707 pmid: 24551500 |
|
(b) Martins, S.; Branco, P. S.; de la Torre, M. C.; Sierra, M. A.; Pereira, A. Synlett 2010, 2918.
pmid: 24551500 |
|
|
(c) Jafarpour, F.; Darvishmolla, M.; Azaddoost, N.; Mohaghegh, F. New J. Chem. 2019, 43, 9328.
doi: 10.1039/c8nj06410e pmid: 24551500 |
|
|
(d) Najib, A.; Tabuchi, S.; Hirano, K.; Miura, M. Heterocycles 2016, 92, 1187.
doi: 10.3987/COM-16-13459 pmid: 24551500 |
|
|
(e) Schroll, P.; Hari, D. P.; König, B. ChemistryOpen 2012, 1, 130.
doi: 10.1002/open.201200011 pmid: 24551500 |
|
| [30] |
(a) Ma, D.; Cai, Q. Acc. Chem. Res. 2008, 41, 1450.
doi: 10.1021/ar8000298 |
|
(b) Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534.
doi: 10.1021/ar800098p |
|
|
(c) Surry, D.; Buchwald, S. Angew. Chem., nt. Ed. 2008, 47, 6338.
doi: 10.1002/anie.v47:34 |
|
| [31] |
(a) He, L. M.; Qiu, G. Y. S.; Gao, Y. Q.; Wu, J. Org. Biomol. Chem. 2014, 12, 6965.
doi: 10.1039/C4OB01286K |
|
(b) Colleville, A. P.; Horan, R. A. J.; Olazabal, S.; Tomkinson, N. C. O. Org. Process Res. Dev. 2016, 20, 1283.
doi: 10.1021/acs.oprd.6b00117 |
|
|
(c) Galli, C. Chem. Rev. 1988, 88, 765.
doi: 10.1021/cr00087a004 |
|
| [32] |
(a) Honraedt, A.; Callonnec, F. L.; Grognec, E. L.; Fernandez, V.; Felpin, F. X. J. Org. Chem. 2013, 78, 4604.
doi: 10.1021/jo4004426 pmid: 21517086 |
|
(b) Susperregui, N.; Miqueu, K.; Sotiropoulos, J. M.; Callonnec, F. L.; Fouquet, E.; Felpin, F. X. Chem.-Eur. J. 2012, 18, 7210.
doi: 10.1002/chem.201200444 pmid: 21517086 |
|
|
(c) Callonnec, F. L.; Fouquet, E.; Felpin, F. X. Org. Lett. 2011, 13, 2646.
doi: 10.1021/ol200752x pmid: 21517086 |
|
| [33] |
(a) Yuan, J. W.; Liu, S. N.; Qu, L. B. Tetrahedron 2017, 73, 2267.
doi: 10.1016/j.tet.2017.03.009 |
|
(b) Yuan, J. W.; Liu, S. N.; Qu, L. B. Adv. Synth. Catal. 2017, 359, 4197.
doi: 10.1002/adsc.v359.23 |
|
|
(c) Yuan, J. W.; Qu, L. B. Chin. Chem. Lett. 2017, 28, 981.
doi: 10.1016/j.cclet.2017.01.016 |
|
|
(d) Yuan, J. W.; Yang, L. R.; Mao, P.; Qu, L. B. Org. Chem. Front. 2017, 4, 545.
doi: 10.1039/C6QO00533K |
|
| [34] |
(a) Bonin, H.; Sauthier, M.; Felpin, F. X. Adv. Synth. Catal. 2014, 356, 645.
doi: 10.1002/adsc.v356.4 |
|
(b) Gowrisankar, S.; Seayad, J. Chem.-Eur. J. 2014, 20, 12754.
doi: 10.1002/chem.201403640 |
|
|
(c) Doyle, M. P.; Siegfried, B.; Elliott, R. C.; Dellaria, J. F. J. Org. Chem. 1977, 42, 2431.
doi: 10.1021/jo00434a018 |
|
|
(d) Obushak, N. D.; Lesyuk, A.; Gorak, Y.; Matiichuk, V. Russ. J. Org. Chem. 2009, 45, 1375.
doi: 10.1134/S1070428009090103 |
|
| [35] |
Cao, Y.; Zhao, H.; Zhang-Negrerie, D.; Du, Y. F.; Zhao, K. Adv. Synth. Catal. 2016, 358, 3610.
doi: 10.1002/adsc.v358.22 |
| [1] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [2] | 曹瑞霞, 贾玉萍. 含香豆素的吡咯并[2,3-d]嘧啶衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3304-3311. |
| [3] | 黄芬, 罗维纬, 周俊. 基于C—H键断裂的多氯烷基化反应研究进展[J]. 有机化学, 2023, 43(7): 2368-2390. |
| [4] | 田钰, 张娟, 高文超, 常宏宏. 二甲亚砜作为甲基化试剂在有机合成中的应用[J]. 有机化学, 2023, 43(7): 2391-2406. |
| [5] | 陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119. |
| [6] | 孔德亮, 戴闻, 赵怡玲, 陈艺林, 朱红平. 脒基胺硼基硅宾与单酮和二酮的氧化环加成反应研究[J]. 有机化学, 2023, 43(5): 1843-1851. |
| [7] | 鲍志成, 李慕尧, 王剑波. 铜催化芳基重氮乙酸酯与双[(频哪醇)硼基]甲烷的偶联反应[J]. 有机化学, 2023, 43(5): 1808-1814. |
| [8] | 李春生, 连晓琪, 陈莲芬. 铜催化亚砜叶立德与邻苯二胺[4+2]环加成反应[J]. 有机化学, 2023, 43(4): 1492-1498. |
| [9] | 刘洋, 黄翔, 王敏, 廖建. 铜催化环酮亚胺与β,γ-不饱和N-酰基吡唑不对称Mannich-Type反应[J]. 有机化学, 2023, 43(4): 1499-1509. |
| [10] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [11] | 刘春阳, 李燕, 张前. 铜催化环状烯烃烯丙位C(sp3)—H磺酰化反应研究[J]. 有机化学, 2023, 43(3): 1091-1101. |
| [12] | 韩彪, 李维双, 陈舒晗, 张泽浪, 赵雪, 张瑶瑶, 朱磊. 铜催化不饱和化合物硅加成反应的研究进展[J]. 有机化学, 2023, 43(2): 555-572. |
| [13] | 陈志远, 杨梦维, 徐建林, 徐允河. 铜催化双炔膦氧化物硅质子化反应合成β-硅基取代的乙烯基膦氧化物[J]. 有机化学, 2023, 43(10): 3598-3607. |
| [14] | 许力, 吕兰兰, 王香善. 铜催化烯醇硅醚与芳基亚磺酸钠合成β-酮砜的研究[J]. 有机化学, 2023, 43(10): 3644-3651. |
| [15] | 胡朝明, 吴纪红, 吴晶晶, 吴范宏. 直接三氟甲硒基化反应研究进展[J]. 有机化学, 2023, 43(1): 36-56. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||