有机化学 ›› 2023, Vol. 43 ›› Issue (5): 1808-1814.DOI: 10.6023/cjoc202301027 上一篇 下一篇
所属专题: 有机硼化学专辑
研究论文
收稿日期:2023-01-31
修回日期:2023-03-12
发布日期:2023-03-23
通讯作者:
王剑波
基金资助:
Zhicheng Bao, Muyao Li, Jianbo Wang( )
)
Received:2023-01-31
Revised:2023-03-12
Published:2023-03-23
Contact:
Jianbo Wang
Supported by:文章分享
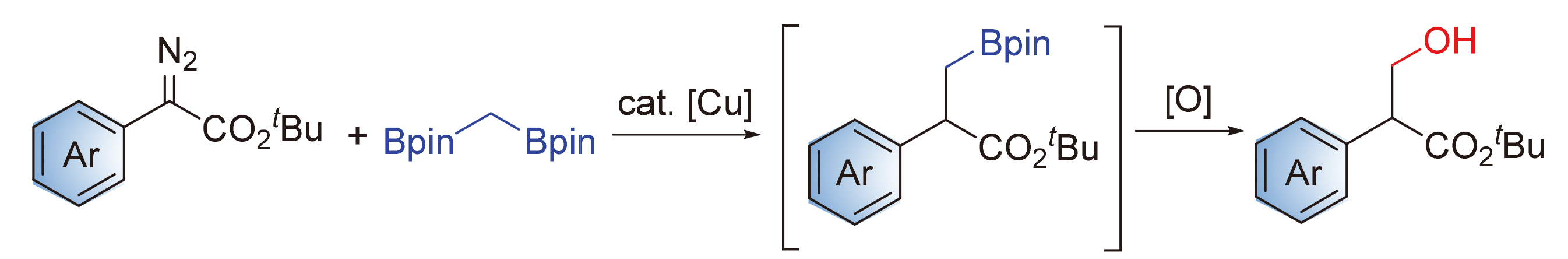
报道铜催化下重氮苯乙酸酯作为卡宾前体与双[(频哪醇)硼基]甲烷的交叉偶联反应, 实现sp3-杂化碳碳键的构筑. 条件筛选表明亚磷酰胺配体monophos对该反应的成功尤为关键. 原位氧化使得硼酯产物以醇的形式被分离, 反应具有高效率以及良好的官能团兼容性. 该两步反应是芳基重氮酯类化合物形式上的羟甲基化.
鲍志成, 李慕尧, 王剑波. 铜催化芳基重氮乙酸酯与双[(频哪醇)硼基]甲烷的偶联反应[J]. 有机化学, 2023, 43(5): 1808-1814.
Zhicheng Bao, Muyao Li, Jianbo Wang. Copper-Catalyzed Cross-Coupling of Aryldiazoacetates with Bis[(pinacolato)boryl]methane[J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1808-1814.
| Entry | [Cu] | Ligand | Base | Solvent | Yieldb/ % |
|---|---|---|---|---|---|
| 1 | CuCl | Ligandc | LiOtBu | Toluene | 0 |
| 2 | CuCl | L1 | LiOtBu | Toluene | 24 |
| 3 | CuCl | L2~L5 | LiOtBu | Toluene | 0 |
| 4 | CuCl | L1 | Based | Toluene | 0 |
| 5 | CuCl | L1 | LiOtBu | THF | 0 |
| 6 | CuCl | L1 | LiOtBu | DCE | 0 |
| 7 | CuCl | L1 | LiOtBu | m-Xylene | 18 |
| 8 | CuCl | L1 | LiOtBu | Chlorobenzene | 14 |
| 9 | CuCl | L1 | LiOtBu | Trifluorotoluene | 8 |
| 10e | CuCl | L1 | LiOtBu | Toluene | 38 |
| 11f | CuCl | L1 | LiOtBu | Toluene | 52 |
| 12 | CuBr | L1 | LiOtBu | Toluene | 30 |
| 13 | CuI | L1 | LiOtBu | Toluene | 24 |
| 14 | CuTC | L1 | LiOtBu | Toluene | 38 |
| 15 | Cu(MeCN)4PF6 | L1 | LiOtBu | Toluene | 48 |
| 16 | CuCl2 | L1 | LiOtBu | Toluene | 48 |
| 17g | CuCl | L1 | LiOtBu | Toluene | 54 |
| 18h | CuCl | L1 | LiOtBu | Toluene | 74 |
| Entry | [Cu] | Ligand | Base | Solvent | Yieldb/ % |
|---|---|---|---|---|---|
| 1 | CuCl | Ligandc | LiOtBu | Toluene | 0 |
| 2 | CuCl | L1 | LiOtBu | Toluene | 24 |
| 3 | CuCl | L2~L5 | LiOtBu | Toluene | 0 |
| 4 | CuCl | L1 | Based | Toluene | 0 |
| 5 | CuCl | L1 | LiOtBu | THF | 0 |
| 6 | CuCl | L1 | LiOtBu | DCE | 0 |
| 7 | CuCl | L1 | LiOtBu | m-Xylene | 18 |
| 8 | CuCl | L1 | LiOtBu | Chlorobenzene | 14 |
| 9 | CuCl | L1 | LiOtBu | Trifluorotoluene | 8 |
| 10e | CuCl | L1 | LiOtBu | Toluene | 38 |
| 11f | CuCl | L1 | LiOtBu | Toluene | 52 |
| 12 | CuBr | L1 | LiOtBu | Toluene | 30 |
| 13 | CuI | L1 | LiOtBu | Toluene | 24 |
| 14 | CuTC | L1 | LiOtBu | Toluene | 38 |
| 15 | Cu(MeCN)4PF6 | L1 | LiOtBu | Toluene | 48 |
| 16 | CuCl2 | L1 | LiOtBu | Toluene | 48 |
| 17g | CuCl | L1 | LiOtBu | Toluene | 54 |
| 18h | CuCl | L1 | LiOtBu | Toluene | 74 |
| [1] |
For selected reviews, see: (a) Liu,Z.; Zhang, Y.; Wang, J. Chin. J. Org. Chem. 2013, 33, 687. (in Chinese)
doi: 10.6023/cjoc201301023 |
|
(刘振兴, 张艳, 王剑波, 有机化学, 2013, 33, 687.)
doi: 10.6023/cjoc201301023 |
|
|
(b) Xia, Y.; Qiu, D.; Wang, J. Chem. Rev. 2017, 117, 13810.
doi: 10.1021/acs.chemrev.7b00382 |
|
|
(c) Liu, Z.; Sivaguru, P.; Zanoni, G.; Bi, X. Acc. Chem. Res. 2022, 55, 1763.
doi: 10.1021/acs.accounts.2c00186 |
|
|
For selected recent examples, see:
|
|
|
(d) Xu, Z.-W.; Zhang, W.; Lin, J.-H.; Jin, C.-M.; Xiao, J.-C. Chin. J. Chem. 2020, 38, 1647.
doi: 10.1002/cjoc.v38.12 |
|
|
(e) Liu, B.; Xu, M.-H. Chin. J. Chem. 2021, 39, 1911.
doi: 10.1002/cjoc.v39.7 |
|
|
(f) Li, F.; Lu, B.; Liu, Y.; Wang, X. Chin. J. Org. Chem. 2022, 42, 3390. (in Chinese)
doi: 10.6023/cjoc202206053 |
|
|
(李芳洁, 卢斌, 刘阳, 王晓明, 有机化学, 2022, 42, 3390.)
doi: 10.6023/cjoc202206053 |
|
|
(g) Li, S.; Zhou, L. Chin. J. Org. Chem. 2022, 42, 3944.
doi: 10.6023/cjoc202206058 |
|
|
(h) Shang, M.; Zhang, L.; Chen, M.; Hu, W.; He, X.; Lu, H. Chin. J. Org. Chem. 2022, 42, 3816. (in Chinese)
doi: 10.6023/cjoc202205039 |
|
|
(商铭洲, 张兰兰, 陈淼淼, 胡汪成, 何心伟, 陆红健, 有机化学, 2022, 42, 3816.)
doi: 10.6023/cjoc202205039 |
|
|
(i) Xia, J.; Gu, Z. Chin. J. Chem. 2020, 38, 1081.
doi: 10.1002/cjoc.v38.10 |
|
| [2] |
For selected examples of alkynes, see: (a) Xiao,Q.; Xia, Y.; Li, H.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2011, 50, 1114.
doi: 10.1002/anie.v50.5 pmid: 21341655 |
|
(b) Ye, F.; Ma, X.; Xiao, Q.; Li, H.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2012, 134, 5742.
doi: 10.1021/ja3004792 pmid: 21341655 |
|
|
(c) Hossain, M. L.; Ye, F.; Zhang, Y.; Wang, J. J. Org. Chem. 2013, 78, 1236.
doi: 10.1021/jo3024686 pmid: 21341655 |
|
|
(d) Ye, F.; Hossain, M. L.; Xu, Y.; Ma, X.; Xiao, Q.; Zhang, Y.; Wang, J. Chem.-Asian J. 2013, 8, 1404.
doi: 10.1002/asia.v8.7 pmid: 21341655 |
|
|
(e) Ye, F.; Wang, C.; Ma, X.; Hossain, M. L.; Xia, Y.; Zhang, Y.; Wang, J. J. Org. Chem. 2015, 80, 647.
doi: 10.1021/jo502316q pmid: 21341655 |
|
|
(f) Poh, J.-S.; Tran, D. N.; Battilocchio, C.; Hawkins, J. M.; Ley, S. V. Angew. Chem., Int. Ed. 2015, 54, 7920.
doi: 10.1002/anie.201501538 pmid: 21341655 |
|
|
(g) Tang, Y.; Chen, Q.; Liu, X.; Wang, G.; Lin, L.; Feng, X. Angew. Chem., Int. Ed. 2015, 54, 9512.
doi: 10.1002/anie.201501918 pmid: 21341655 |
|
|
(h) Chu, W.-D.; Zhang, L.; Zhang, Z.; Zhou, Q.; Mo, F.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2016, 138, 14558.
doi: 10.1021/jacs.6b09674 pmid: 21341655 |
|
|
For selected examples of arenes, see: (i) Zhao, X.; Wu, G.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2011, 133, 3296.
doi: 10.1021/ja111249p pmid: 21341655 |
|
|
(j) Xiao, Q.; Ling, L.; Ye, F.; Tan, R.; Tian, L.; Zhang, Y.; Li, Y.; Wang, J. J. Org. Chem. 2013, 78, 3879.
doi: 10.1021/jo4002883 pmid: 21341655 |
|
|
(k) Xu, S.; Wu, G.; Ye, F.; Wang, X.; Li, H.; Zhao, X.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2015, 54, 4669.
doi: 10.1002/anie.201412450 pmid: 21341655 |
|
| [3] |
For selected examples, see: (a) Hu,M.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2012, 134, 15257.
doi: 10.1021/ja307058c pmid: 28276608 |
|
(b) Hu, M.; He, Z.; Gao, B.; Li, L.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2013, 135, 17302.
doi: 10.1021/ja409941r pmid: 28276608 |
|
|
(c) Wang, X.; Zhou, Y.; Ji, G.; Wu, G.; Li, M.; Zhang, Y.; Wang, J. Eur. J. Org. Chem. 2014, 3093.
pmid: 28276608 |
|
|
(d) Das, A.; Wang, D.; Belhomme, M. C.; Szabó, K. J. Org. Lett. 2015, 17, 4754.
doi: 10.1021/acs.orglett.5b02285 pmid: 28276608 |
|
|
(e) Xu, S.; Gao, Y.; Chen, R.; Wang, K.; Zhang, Y.; Wang, J. Chem. Commun. 2016, 52, 4478.
doi: 10.1039/C6CC00444J pmid: 28276608 |
|
|
(f) Zhang, H.; Wu, G.; Yi, H.; Sun, T.; Wang, B.; Zhang, Y.; Dong, G.; Wang, J. Angew. Chem., Int. Ed. 2017, 56, 3945.
doi: 10.1002/anie.201612138 pmid: 28276608 |
|
| [4] |
For selected reviews, see: (a) Paul,S.; Das, K. K.; Aich, D.; Manna, S.; Panda, S. Org. Chem. Front. 2022, 9, 838.
doi: 10.1039/D1QO01300A pmid: 29379940 |
|
(b) Lee, Y.; Han, S.; Cho, S. H. Acc. Chem. Res. 2021, 54, 3917.
doi: 10.1021/acs.accounts.1c00455 pmid: 29379940 |
|
|
(c) Nallagonda, R.; Padala, K.; Masarwa, A. Org. Biomol. Chem. 2018, 16, 1050.
doi: 10.1039/c7ob02978k pmid: 29379940 |
|
|
(d) Miralles, N.; Maza, R. J.; Fernández, E. Adv. Synth. Catal. 2018, 360, 1306.
doi: 10.1002/adsc.v360.7 pmid: 29379940 |
|
|
(e) Wu, C.; Wang, J. Tetrahedron Lett. 2018, 59, 2128.
doi: 10.1016/j.tetlet.2018.04.061 pmid: 29379940 |
|
|
(f) Jo, W.; Lee, J. H.; Cho, S. H. Chem. Commun. 2021, 57, 4346.
doi: 10.1039/D1CC01048D pmid: 29379940 |
|
|
(g) Xu, L.; Zhang, S.; Li, P. Chem. Soc. Rev. 2015, 44, 8848.
doi: 10.1039/C5CS00338E pmid: 29379940 |
|
| [5] |
Li, H.; Shangguan, X.; Zhang, Z.; Huang, S.; Zhang, Y.; Wang, J. Org. Lett. 2014, 16, 448.
doi: 10.1021/ol403338s pmid: 24350698 |
| [6] |
For selected examples, see: (a) Wu,C.; Bao, Z.; Dou, B.; Wang, J. Chem.-Eur. J. 2021, 27, 2294.
doi: 10.1002/chem.v27.7 |
|
(b) Bao, Z.; Wu, C.; Wang, J. Eur. J. Org. Chem. 2022, e202201264.
|
|
|
(c) Bao, Z.; Huang, M.; Xu, Y.; Zhang, X.; Wu, Y.-D.; Wang, J. Angew. Chem., Int. Ed. 2023, 62, e202216356.
doi: 10.1002/anie.v62.9 |
|
| [7] |
Walton, J. C.; McCarroll, A. J.; Chen, Q.; Carboni, B.; Nziengui, R. J. Am. Chem. Soc. 2000, 122, 5455.
doi: 10.1021/ja9944812 |
| [8] |
Huang, Z.; Hartwig, J. F. Angew. Chem., Int. Ed. 2012, 51, 1028.
doi: 10.1002/anie.v51.4 |
| [1] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [2] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [3] | 陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119. |
| [4] | 李春生, 连晓琪, 陈莲芬. 铜催化亚砜叶立德与邻苯二胺[4+2]环加成反应[J]. 有机化学, 2023, 43(4): 1492-1498. |
| [5] | 刘洋, 黄翔, 王敏, 廖建. 铜催化环酮亚胺与β,γ-不饱和N-酰基吡唑不对称Mannich-Type反应[J]. 有机化学, 2023, 43(4): 1499-1509. |
| [6] | 刘春阳, 李燕, 张前. 铜催化环状烯烃烯丙位C(sp3)—H磺酰化反应研究[J]. 有机化学, 2023, 43(3): 1091-1101. |
| [7] | 韩彪, 李维双, 陈舒晗, 张泽浪, 赵雪, 张瑶瑶, 朱磊. 铜催化不饱和化合物硅加成反应的研究进展[J]. 有机化学, 2023, 43(2): 555-572. |
| [8] | 陈志远, 杨梦维, 徐建林, 徐允河. 铜催化双炔膦氧化物硅质子化反应合成β-硅基取代的乙烯基膦氧化物[J]. 有机化学, 2023, 43(10): 3598-3607. |
| [9] | 许力, 吕兰兰, 王香善. 铜催化烯醇硅醚与芳基亚磺酸钠合成β-酮砜的研究[J]. 有机化学, 2023, 43(10): 3644-3651. |
| [10] | 陈飞, 陶晟, 刘宁, 代斌. CNN型双核Cu(I)配合物室温催化固定CO2的直接羧基化反应[J]. 有机化学, 2022, 42(8): 2471-2480. |
| [11] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| [12] | 孙天义, 张依凡, 孟远倢, 王怡, 朱琦峰, 姜玉新, 刘石惠. 可见光-铜共催化的糖类区域选择性氧烷基化反应[J]. 有机化学, 2022, 42(5): 1414-1422. |
| [13] | 孙亚敏, 李锡勇, 袁金伟, 余加琳, 刘帅楠. 温和条件下以芳基胺为原料CuI催化下区域选择性合成3-芳基香豆素[J]. 有机化学, 2022, 42(2): 631-640. |
| [14] | 谢阳, 宣俊. 重氮化合物作为自由基前体参与的光催化反应[J]. 有机化学, 2022, 42(12): 4247-4256. |
| [15] | 李森, 周磊. 可见光促进重氮化合物参与的自由基反应[J]. 有机化学, 2022, 42(12): 3944-3958. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||