有机化学 ›› 2022, Vol. 42 ›› Issue (9): 2814-2822.DOI: 10.6023/cjoc202204057 上一篇 下一篇
研究论文
贾润红a,*( ), 刘帅b, 王世超b, 郝文娟b, 姜波b,*(
), 刘帅b, 王世超b, 郝文娟b, 姜波b,*( )
)
收稿日期:2022-04-24
修回日期:2022-06-16
发布日期:2022-06-29
通讯作者:
贾润红, 姜波
基金资助:
Runhong Jiaa( ), Shuai Liub, Shichao Wangb, Wenjuan Haob, Bo Jiangb(
), Shuai Liub, Shichao Wangb, Wenjuan Haob, Bo Jiangb( )
)
Received:2022-04-24
Revised:2022-06-16
Published:2022-06-29
Contact:
Runhong Jia, Bo Jiang
Supported by:文章分享
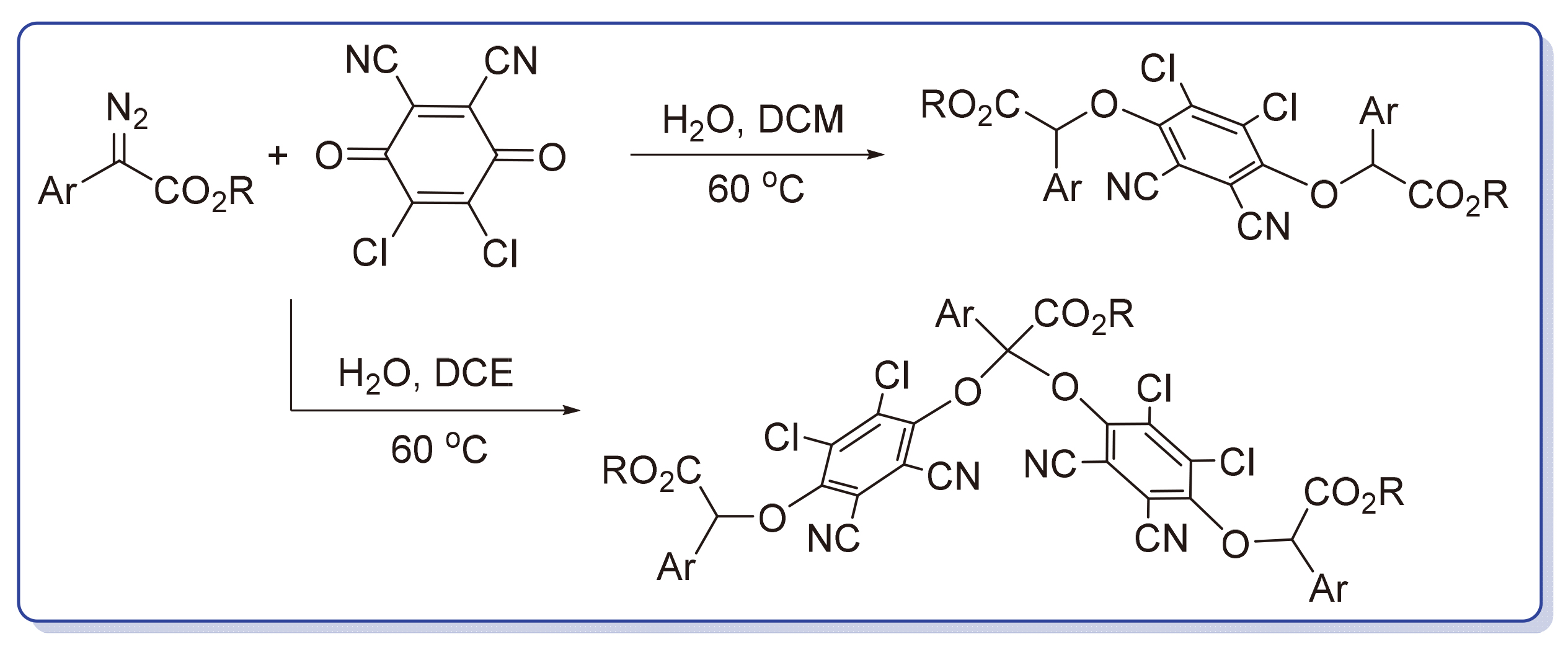
报道了一类新型无金属、溶剂调控的2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)与α-芳基α-重氮酸酯的去重氮化氧合反应. 当反应使用二氯甲烷作溶剂时, DDQ与α-芳基α-重氮酯以物质的量比1∶2发生双去重氮化氧氢键插入反应, 得到α,α'-(1,4-苯二氧基)二酯衍生物为主要产物, 而使用1,2-二氯乙烷时, 该反应底物以物质的量比2∶3进行反应, 经双去重氮化氧氢键插入和去重氮化双氧合反应串联过程合成了一系列结构复杂的α,α-双芳氧基酯衍生物, 收率中等至良好. 控制实验证实了氧氢插入过程中的氢元素来自于溶剂中的水分子. 此外, 该反应无需金属催化剂, 具有条件温和、操作简便以及化学选择性可控等优点.
贾润红, 刘帅, 王世超, 郝文娟, 姜波. 溶剂调控的α-芳基α-重氮酯与2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)的去重氮化氧合反应[J]. 有机化学, 2022, 42(9): 2814-2822.
Runhong Jia, Shuai Liu, Shichao Wang, Wenjuan Hao, Bo Jiang. A Solvent-Regulated Dediazotized Oxygenation of α-Aryl α-Diazoesters and 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)[J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2814-2822.
| Entry | 1a/2 (molar ratio) | Cat. (mol%) | H2O/equiv. | Solvent | t/℃ | Yieldc/% | |
|---|---|---|---|---|---|---|---|
| 3a | 4a | ||||||
| 1 | 1.5∶1 | RhOAc (1.0) | — | DCM | 60 | 30 | 31 |
| 2 | 2∶1 | RhOAc (1.0) | — | DCM | 60 | 32 | 37 |
| 3 | 3∶1 | RhOAc (1.0) | — | DCM | 60 | 60 | 21 |
| 4 | 3∶1 | — | — | DCM | 60 | 60 | 19 |
| 5 | 3∶1 | — | — | DCM | r.t. | 34 | 33 |
| 6 | 3∶1 | — | — | DCM | 40 | 46 | 36 |
| 7 | 3∶1 | — | — | DCM | 80 | 48 | Trace |
| 8 | 3∶1 | — | — | DCE | 60 | Trace | 53 |
| 9 | 3∶1 | — | — | 1,4-Dioxane | 60 | Trace | Trace |
| 10 | 3∶1 | — | — | THF | 60 | Trace | Trace |
| 11 | 3∶1 | — | — | DMF | 60 | Trace | Trace |
| 12 | 4∶1 | — | — | DCE | 60 | Trace | 62 |
| 13 | 4∶1 | — | — | DCE | 40 | 26 | 46 |
| 14 | 4∶1 | — | — | DCE | 80 | Trace | 56 |
| 15 | 3∶1 | — | 1.0 | Dry DCM | 60 | 45 | 23 |
| 16 | 3∶1 | — | 2.0 | Dry DCM | 60 | 61 | 22 |
| 17 | 3∶1 | — | 3.0 | Dry DCM | 60 | 56 | 32 |
| 18 | 4∶1 | — | 2.0 | Dry DCE | 60 | Trace | 61 |
| Entry | 1a/2 (molar ratio) | Cat. (mol%) | H2O/equiv. | Solvent | t/℃ | Yieldc/% | |
|---|---|---|---|---|---|---|---|
| 3a | 4a | ||||||
| 1 | 1.5∶1 | RhOAc (1.0) | — | DCM | 60 | 30 | 31 |
| 2 | 2∶1 | RhOAc (1.0) | — | DCM | 60 | 32 | 37 |
| 3 | 3∶1 | RhOAc (1.0) | — | DCM | 60 | 60 | 21 |
| 4 | 3∶1 | — | — | DCM | 60 | 60 | 19 |
| 5 | 3∶1 | — | — | DCM | r.t. | 34 | 33 |
| 6 | 3∶1 | — | — | DCM | 40 | 46 | 36 |
| 7 | 3∶1 | — | — | DCM | 80 | 48 | Trace |
| 8 | 3∶1 | — | — | DCE | 60 | Trace | 53 |
| 9 | 3∶1 | — | — | 1,4-Dioxane | 60 | Trace | Trace |
| 10 | 3∶1 | — | — | THF | 60 | Trace | Trace |
| 11 | 3∶1 | — | — | DMF | 60 | Trace | Trace |
| 12 | 4∶1 | — | — | DCE | 60 | Trace | 62 |
| 13 | 4∶1 | — | — | DCE | 40 | 26 | 46 |
| 14 | 4∶1 | — | — | DCE | 80 | Trace | 56 |
| 15 | 3∶1 | — | 1.0 | Dry DCM | 60 | 45 | 23 |
| 16 | 3∶1 | — | 2.0 | Dry DCM | 60 | 61 | 22 |
| 17 | 3∶1 | — | 3.0 | Dry DCM | 60 | 56 | 32 |
| 18 | 4∶1 | — | 2.0 | Dry DCE | 60 | Trace | 61 |
| [1] |
(a) Guan, R.; Bennett, E. L.; Huang, Z.; Xiao, J. Green Chem. 2022, 24, 2946.
doi: 10.1039/D1GC04603A |
|
(b) Zhang, Y.; Chi, Z.; Li, X.; Xie, Z. Chin. J. Chem. 2022, 40, 183.
doi: 10.1002/cjoc.202100737 |
|
|
(c) Wang, Y.-H.; Yang, Q.; Walsh, P. J.; Schelter, E. J. Org. Chem. Front. 2022, 9, 2612.
doi: 10.1039/D2QO00362G |
|
|
(d) Han, T.; Jiang, Y.; Ji, X.; Deng, G.-J.; Huang, H. Org. Chem. Front. 2020, 7, 1671.
doi: 10.1039/D0QO00233J |
|
|
(e) Vijaykumar, M.; Punji, B. Synthesis 2021, 53, 2935.
doi: 10.1055/a-1481-2584 |
|
|
(f) Hu, D.; Jiang, X. Green Chem. 2022, 24, 124.
doi: 10.1039/D1GC04042A |
|
| [2] |
(a) Dong, S.; Liu, X.; Feng, X. Acc. Chem. Res. 2022, 55, 415.
doi: 10.1021/acs.accounts.1c00664 |
|
(b) Zhang, Z.; Yu, Y.; Huang, F.; Yi, X.; Xu, Y.; He, Y.; Baell, J. B.; Huang, H. Green Chem. 2020, 22, 1594.
doi: 10.1039/C9GC03428E |
|
|
(c) Zhu, S.-F.; Zhou, Q.-L. Acc. Chem. Res. 2012, 45, 1365.
doi: 10.1021/ar300051u |
|
|
(d) Zhao, J.; Ji, S.; Guo, C.; Li, H.; Dong, J.; Guo, P.; Wang, D.; Li, Y.; Toste, F. D. Nat. Catal. 2021, 4, 523-531.
doi: 10.1038/s41929-021-00637-7 |
|
| [3] |
Harada, S.; Tanikawa, K.; Homma, H.; Sakai, C.; Ito, T.; Nemoto, T. Chem.-Eur. J. 2019, 25, 12058.
doi: 10.1002/chem.201902126 |
| [4] |
Song, X.-G.; Zhu, S.-F.; Xie, X.-L.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2013, 52, 2555.
doi: 10.1002/anie.201209455 |
| [5] |
Xie, X.-L.; Zhu, S.-F.; Guo, J.-X.; Cai, Y.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2014, 53, 2978.
doi: 10.1002/anie.201309820 |
| [6] |
(a) Hong, K.; Dong, S.; Xu, X.; Zhang, Z.; Shi, T.; Yuan, H.; Xu, X.; Hu, W. ACS Catal. 2021, 11, 675.
|
|
(b) Dong, C.; Zhang, C.; Wang, X.; Shen, R. Asian J. Org. Chem. 2021, 10, 1514.
doi: 10.1002/ajoc.202100195 |
|
|
(c) Li, Y.; Zhao, Y.-T.; Zhou, T.; Chen, M.-Q.; Li, Y.-P.; Huang, M.-Y.; Xu, Z.-C.; Zhu, S.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2020, 142, 10557.
doi: 10.1021/jacs.0c04532 |
|
| [7] |
(a) Jiang, Y.; Xu, K.; Zeng, C. Chem. Rev. 2018, 118, 4485.
doi: 10.1021/acs.chemrev.7b00271 |
|
(b) Wang, L.; Zhang, Y.; Miao, A.-Q.; Zhang, T.-S.; Wang, X.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Chem. Commun. 2022, 58, 4376.
doi: 10.1039/D2CC00206J |
|
|
(c) Zhang, T.-S.; Hao, W.-J.; Wang, R.; Wang, S.-C.; Tu, S.-J.; Jiang, B. Green Chem. 2020, 22, 4259.
doi: 10.1039/D0GC00771D |
|
|
(d) Wen, L.-R.; Wang, N.-N.; Du, W.-B.; Zhu, M.-Z.; Pan, C.; Zhang, L.-B.; Li, M. Chin. J. Chem. 2021, 39, 1831.
doi: 10.1002/cjoc.202100132 |
|
|
(e) Jiang, P.; Shan, Z.; Chen, S.; Wang, Q.; Jiang, S.; Zheng, H.; Deng, G.-J. Chin. J. Chem. 2022, 40, 365.
doi: 10.1002/cjoc.202100670 |
|
|
(f) Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Liu, H.-Y.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907.
doi: 10.1016/j.cclet.2021.01.021 |
|
|
(g) Wu, Z.-L.; Chen, J.-Y.; Tian, X.-Z.; Ouyang, W.-T.; Zhang, Z.-T.; He, W.-M. Chin. Chem. Lett. 2022, 33, 1501.
doi: 10.1016/j.cclet.2021.08.071 |
|
|
(h) Jiang, J.; Wang, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1591.
doi: 10.1016/j.cclet.2021.02.067 |
|
| [8] |
(a) Ma, C.; Fang, P.; Liu, Z.-R.; Xu, S.-S.; Xu, K.; Cheng, X.; Lei, A.; Xu, H.-C.; Zeng, C.; Mei, T.-S. Sci. Bull. 2021, 66, 2412.
doi: 10.1016/j.scib.2021.07.011 |
|
(b) Lee, Y.; Han, S.; Cho, S. H. Acc. Chem. Res. 2021, 54, 3917.
doi: 10.1021/acs.accounts.1c00455 |
|
|
(c) Wang, X.; Chen, J.; Ma, N.; Cong, Z. Acta Chim. Sinica 2020, 78, 490. (in Chinese)
doi: 10.6023/A20030086 |
|
|
(王曦翎, 陈杰, 马娜娜, 丛志奇, 化学学报, 2020, 78, 490.)
|
|
|
(d) Lu, Z.; Li, T.; Mudshinge, S. R.; Xu, Bo.; Hammond, G. B. Chem. Rev. 2021, 121, 8452.
doi: 10.1021/acs.chemrev.0c00713 |
|
| [9] |
(a) Zhou, S.; Cai, B.; Hu, C.; Cheng, X.; Li, L.; Xuan, J. Chin. Chem. Lett. 2021, 32, 2577.
doi: 10.1016/j.cclet.2021.03.010 pmid: 33534572 |
|
(b) Leng, H.; Zhao, Q.; Mao, Q.; Liu, S.; Luo, M.; Qin, R.; Huang, W.; Zhan, G. Chin. Chem. Lett. 2021, 32, 2567.
doi: 10.1016/j.cclet.2021.03.009 pmid: 33534572 |
|
|
(c) Zhou, Y.; Chen, H.; Lei, P.; Gui, C.; Wang, H.; Yan, Q.; Wang, W.; Chen, F. Chin. Chem. Lett. 2022, DOI: 10.1016/j.cclet.2022.02.029.
doi: 10.1016/j.cclet.2022.02.029 pmid: 33534572 |
|
|
(d) Muthukrishnan, I.; Karuppasamy, M.; Vachan, B. S.; Rajput, D.; Subbiah, N.; Maheswari, C. U.; Sridharan, V. Org. Chem. Front. 2020, 7, 1616.
doi: 10.1039/D0QO00449A pmid: 33534572 |
|
|
(e) Dong, J.; Wang, L.; Li, H.; Leng, X.; Guo, X.; Hu, Z.; Xu, X. Org. Chem. Front. 2021, 8, 2595.
doi: 10.1039/D1QO00132A pmid: 33534572 |
|
|
(f) Zhang, J.; Shi, S.-Q.; Hao, W.-J.; Dong, G.-Y.; Tu, S.-J.; Jiang, B. J. Org. Chem. 2021, 86, 15886.
doi: 10.1021/acs.joc.0c02898 pmid: 33534572 |
|
| [10] |
Liu, B.-X.; Yan, R.-J.; Du, W.; Chen, Y.-C. Chin. J. Chem. 2022, 40, 1185.
doi: 10.1002/cjoc.202200003 |
| [11] |
He, Y.; Zheng, Z.; Liu, Q.; Zhang, X.; Fan, X. Org. Lett. 2020, 22, 9053.
doi: 10.1021/acs.orglett.0c03442 |
| [12] |
Zhu, C.-F.; Zhang, J.; Zhu, Y.-L.; Hao, W.-J.; Tu, S.-J.; Wang, D.-C.; Jiang, B. Org. Chem. Front. 2021, 8, 1952.
doi: 10.1039/D1QO00124H |
| [13] |
(a) Wang, N.-N.; Hao, W.-J.; Zhang, T.-S.; Li, G.; Wu, Y.-N.; Tu, S.-J.; Jiang, B. Chem. Commun. 2016, 52, 5144.
doi: 10.1039/C6CC00816J |
|
(b) Zhang, T.-S.; Hao, W.-J.; Wang, N.-N.; Li, G.; Jiang, D.-F.; Tu, S.-J.; Jiang, B. Org. Lett. 2016, 18, 3078.
doi: 10.1021/acs.orglett.6b01189 |
|
| [14] |
Ma, G.-H.; Jiang, B.; Tu, X.-J.; Ning, Y.; Tu, S.-J.; Li, G. Org. Lett. 2014, 16, 4504.
doi: 10.1021/ol502048e |
| [15] |
Oshima, T.; Nagai, T. Bull. Chem. Soc. Jpn. 1986, 59, 3865.
doi: 10.1246/bcsj.59.3865 |
| [16] |
(a) Empel, C.; Jana, S.; Pei, C.; Nguyen, T. V.; Koenigs, R. M. Org. Lett. 2020, 22, 7225.
doi: 10.1021/acs.orglett.0c02564 |
|
(b) Zhang, Z.; He, Z.; Xie, Y.; He, T.; Fu, Y.; Yu, Y.; Huang, F. Org. Chem. Front. 2021, 8, 1233.
doi: 10.1039/D0QO01401J |
|
|
(c) Zhou, S.; Cai, B.; Hu, C.; Cheng, X.; Li, L.; Xuan, J. Chin. Chem. Lett. 2021, 32, 2577.
doi: 10.1016/j.cclet.2021.03.010 |
| [1] | 刘腾. 醌亚胺单缩酮的合成以及应用研究进展[J]. 有机化学, 2020, 40(9): 2678-2691. |
| [2] | 张璐, 刘爱芹, 刘华铮, 万仁忠, 孙书涛, 刘磊. 基于化学和对映选择性转移氢化的β,γ-炔基α-氨基酸酯的催化不对称合成研究[J]. 有机化学, 2020, 40(9): 2904-2911. |
| [3] | 从屹康, 曾祥华. 纳米Cu-CuFe2O4在乙醇中催化选择性还原α,β,γ,δ-不饱和羰基化合物[J]. 有机化学, 2020, 40(8): 2411-2418. |
| [4] | 曹姗姗, 刘兆洪, 袁海艳, 杨柳, 张景萍, 毕锡和. 催化剂调控的金属卡宾与1,3-二羰基化合物选择性C—C键与C—H键插入反应机理的理论研究[J]. 有机化学, 2020, 40(8): 2468-2475. |
| [5] | 李庆雪, 李梦伟, 时绍青, 季晓霜, 何春兰, 姜波, 郝文娟. 醋酸碘苯介导的α-重氮羰基化合物的去重氮双氧合反应[J]. 有机化学, 2020, 40(2): 384-390. |
| [6] | 唐子龙, 汪明, 姚园, 谭经照, 代宁宁, 李新兴, 彭丽芬, 焦银春. 羟基取代乙二胺衍生物与醛反应化学选择性合成取代苯并噁嗪和咪唑啉[J]. 有机化学, 2019, 39(3): 800-810. |
| [7] | 杨靖亚, 李娜娜, 周红艳, 李天媛, 谢栋泰, 李政. 2-巯基嘧啶与碳酸Morita-Baylis-Hillman酯的选择性S-烯丙基烷基化[J]. 有机化学, 2017, 37(8): 2078-2085. |
| [8] | 程骁恺, 胡新根, 陆展. 可见光促进氧气参与的均相氧合反应[J]. 有机化学, 2017, 37(2): 251-266. |
| [9] | 程晓红, 钟志成, 叶婷婷, 张冰洁b. 反应型化学传感器在阴离子检测中的应用[J]. 有机化学, 2016, 36(12): 2822-2842. |
| [10] | 李文玲, 吕腾, 杨玉华, 杨亚东. 白藜芦醇低聚物的化学选择性合成[J]. 有机化学, 2013, 33(12): 2443-2459. |
| [11] | 马欣, 李万方, 范为正, 陶晓明, 李晓明, 姚莹, 诸吕锋, 陈厚和, 谢小敏, 张兆国. 多官能团羰基化合物的高选择性不对称氢化[J]. 有机化学, 2012, 32(08): 1353-1358. |
| [12] | 程金生,赵进,黄锁义,江焕峰c. 溶剂对钯催化的叔丁基乙炔低聚反应化学选择性的调控作用[J]. 有机化学, 2007, 27(05): 656-662. |
| [13] | 石启英. Pd(PPh3)2Cl2-CuCl体系催化有机高价碘杂环化合物与末端炔烃的化学选择性交叉偶联反应[J]. 有机化学, 2004, 24(8): 912-915. |
| [14] | 廖联安,李正名. 在水介质中进行的Barbier-Grignard反应[J]. 有机化学, 2000, 20(3): 306-318. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||