有机化学 ›› 2023, Vol. 43 ›› Issue (1): 285-294.DOI: 10.6023/cjoc202206027 上一篇 下一篇
研究论文
凌琳a, 王健b, 李婧c, 李玉学a,*( ), 吕龙a,*(
), 吕龙a,*( )
)
收稿日期:2022-06-17
修回日期:2022-07-20
发布日期:2022-08-17
通讯作者:
李玉学, 吕龙
基金资助:
Lin Linga, Jian Wangb, Jing Lic, Yuxue Lia( ), Long Lua(
), Long Lua( )
)
Received:2022-06-17
Revised:2022-07-20
Published:2022-08-17
Contact:
Yuxue Li, Long Lu
Supported by:文章分享

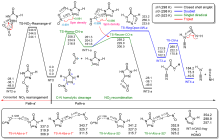
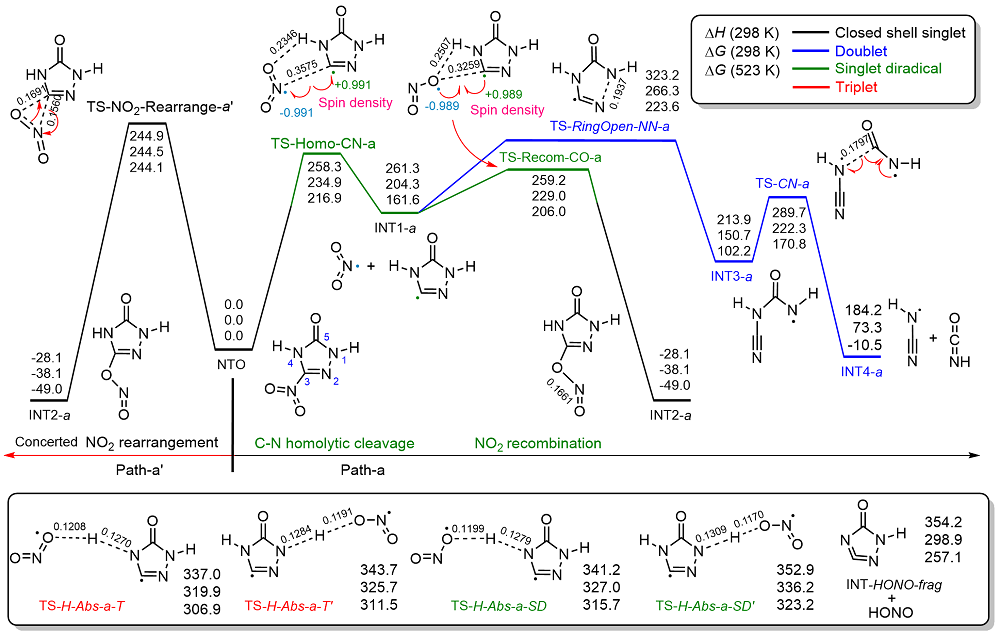
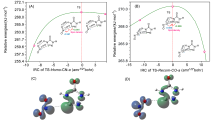
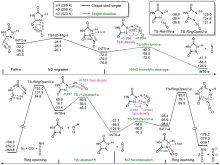
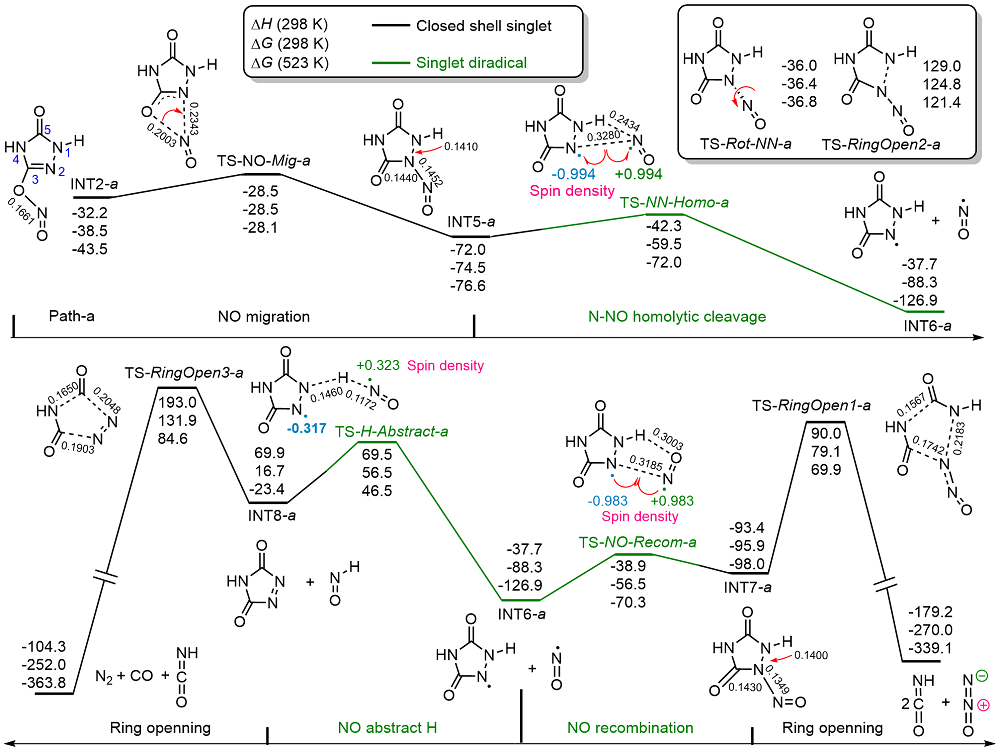
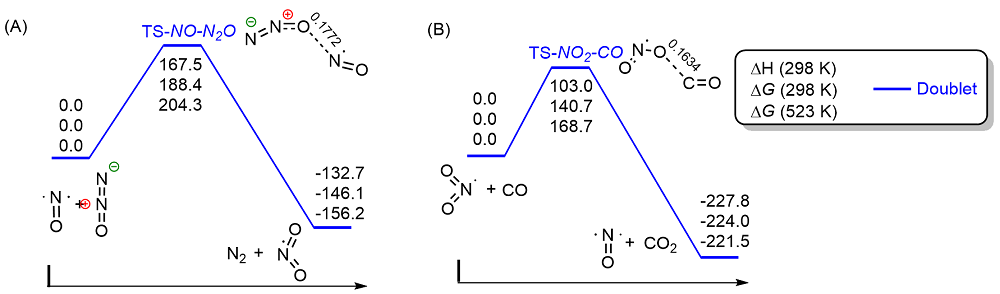
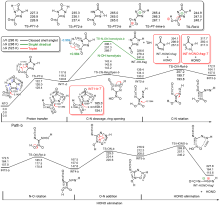
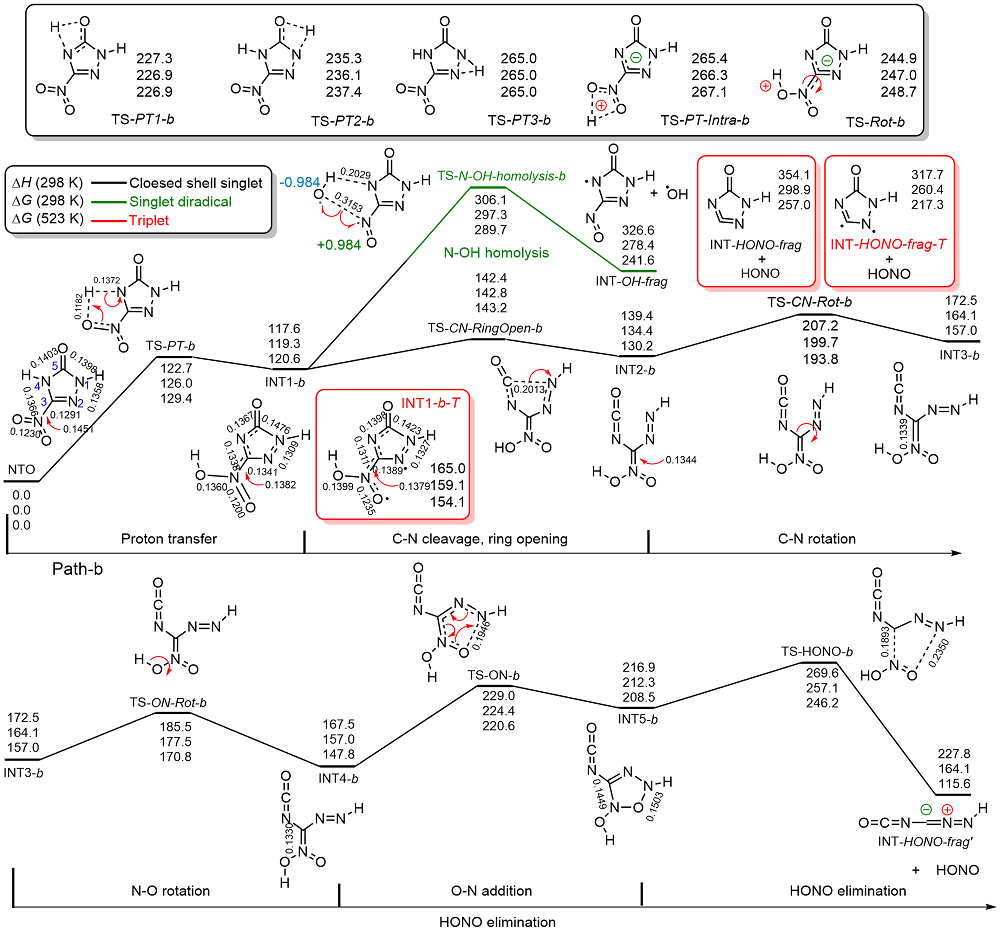
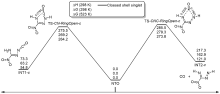
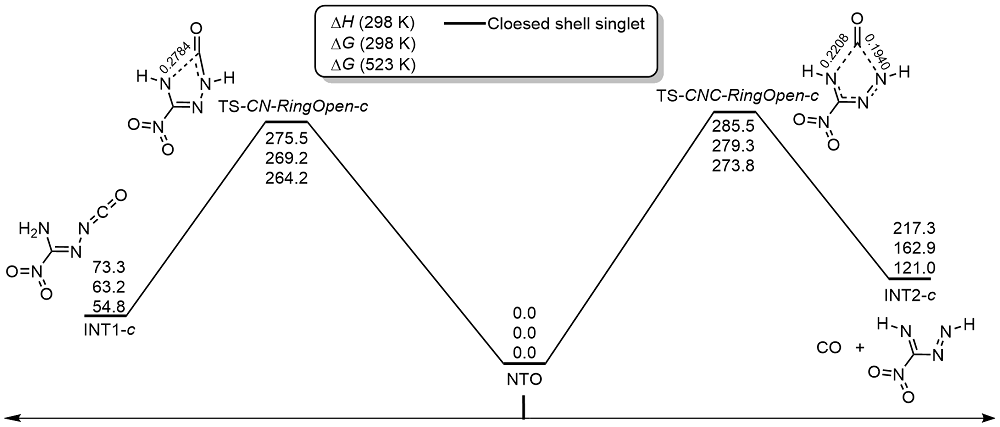
共价键均裂在含能分子的热分解过程中普遍存在, 因此极为重要. 然而, 以往的理论研究通常用键能(ΔH, BDE)估算均裂能垒, 因忽略了熵效应, 必然造成较大误差. 采用对称破缺密度泛函方法(BS-UB3LYP/6-311+G**), 对含能分子3-硝基-1,2,4-三唑-5-酮(NTO)的热分解机理进行了系统研究和梳理, 计算了共价键均裂的过渡态及能垒. 结果表明, C—NO2键均裂和随后的自由基复合是最优途径, 能垒为216.9 kJ•mol–1 (523 K). 随后产生的NO自由基通过多次“复合-均裂”过程促进三唑中间体开环, 分解为HNCO, N2O和CO等小分子, 它们相互反应又生成NO2, N2和CO2. 这些分解产物与诸多实验观测结果一致.
凌琳, 王健, 李婧, 李玉学, 吕龙. 3-硝基-1,2,4-三唑-5-酮(NTO)热分解机理的对称破缺密度泛函理论研究[J]. 有机化学, 2023, 43(1): 285-294.
Lin Ling, Jian Wang, Jing Li, Yuxue Li, Long Lu. Broken-Symmetry Density Functional Theory Study on Pyrolysis Mechanisms of 3-Nitro-1,2,4-triazol-5-one (NTO)[J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 285-294.

| [1] |
Viswanath, D. S.; Ghosh, T. K.; Boddu, V. M. Emerging Energetic Materials: Synthesis, Physicochemical, and Detonation Properties, Springer, Dordrecht, 2018, pp. 163-211.
|
| [2] |
Lee, K.; Chapman, L. B.; Cobura, M. D. J. Energ. Mater. 1987, 5, 27.
doi: 10.1080/07370658708012347 |
| [3] |
Ma, H.-X.; Song, J.-R.; Hu, R.-Z. Chin. J. Expl. Propell. 2006, 29, 9. (in Chinese)
|
|
(马海霞, 宋纪蓉, 胡荣祖, 火炸药学报, 2006, 29, 9.)
|
|
| [4] |
Rothgery, E. F.; Audette, D. E.; Wedlich, R. C.; Csejka, D. A. Thermochim. Acta 1991, 185, 235.
doi: 10.1016/0040-6031(91)80045-K |
| [5] |
Menapace, J. A.; Marlin, J. E.; Bruss, D. R.; Dascher, R. V. J. Phys. Chem. 1991, 95, 5509.
doi: 10.1021/j100167a028 |
| [6] |
Östmark, H.; Bergman, H.; Åqvist, G. Thermochim. Acta 1993, 213, 165.
doi: 10.1016/0040-6031(93)80014-2 |
| [7] |
Prabhakaran, K. V.; Naidu, S. R.; Kurian, E. M. Thermochim. Acta 1994, 241, 199.
doi: 10.1016/0040-6031(94)87018-7 |
| [8] |
Brill, T. B.; Gongwer, P. E.; Williams, G. K. J. Phys. Chem. 1994, 98, 12242.
doi: 10.1021/j100098a020 |
| [9] |
Hara, Y.; Taniguchi, H.; Ikeda, Y.; Takayama, S.; Nakamura, H. Kayaku Gakkaishi 1994, 55, 183.
|
| [10] |
Williams, G. K.; Palopoli, S. F.; Brill, T. B. Combust. Flame 1994, 98, 197.
doi: 10.1016/0010-2180(94)90235-6 |
| [11] |
Oxley, J. C.; Smith, J. L.; Zhou, Z.; McKenney, R. L. J. Phys. Chem. 1995, 99, 10383.
doi: 10.1021/j100025a047 |
| [12] |
Williams, G. K.; Brill, T. B. J. Phys. Chem. 1995, 99, 12536.
doi: 10.1021/j100033a027 |
|
Botcher, T. R.; Beardall, D. J.; Wight, C. A. J. Phys. Chem. 1996, 100, 8802.
|
|
| [13] |
Mcmillen, D. F.; Erlich, D. C.; He, C.; Becker, C. H.; Shockey, D. A. Combust. Flame 1997, 111, 133.
doi: 10.1016/S0010-2180(97)00100-4 |
| [14] |
Long, G. T.; Brems, B. A.; Wight, C. A. J. Phys. Chem. B 2002, 106, 4022.
doi: 10.1021/jp012894v |
| [15] |
Kondrikov, B. N.; Smirnov, S. P.; Minakin, A. V. Propellants, Explos., Pyrotechnics 2004, 29, 27.
|
| [16] |
Sinditskii, V. P.; Smirnov, S. P.; Egorshev, V. Y. Propellants, Explos., Pyrotechnics 2007, 32, 277.
doi: 10.1002/prep.200700029 |
| [17] |
Oxley, J. C.; Smith, J. L.; Rogers, E.; Dong, X. X. J. Phys. Chem. A 1997, 101, 3531.
doi: 10.1021/jp9640078 |
| [18] |
Wang, K.; Wang, J.-L.; Xu, D.; Guo, T.-J.; Wang, W.; Tu, J. Acta Armamentarii 2018, 39, 1727. (in Chinese)
|
|
(王凯, 王俊林, 徐东, 郭天吉, 王伟, 涂建, 兵工学报, 2018, 39, 1727.)
doi: 10.3969/j.issn.1000-1093.2018.09.008 |
|
| [19] |
Harris, N. J.; Lammertsma, K. J. Am. Chem. Soc. 1996, 118, 8048.
doi: 10.1021/ja960834a |
| [20] |
Meredith, C.; Russell, T. P.; Mowrey, R. C.; McDonald, J. R. J. Phys. Chem. A 1998, 102, 471.
doi: 10.1021/jp972602j |
| [21] |
Wang, Y.-M.; Chen, C.; Lin, S.-T. J. Mol. Struct.: Theochem. 1999, 460, 79.
doi: 10.1016/S0166-1280(98)00308-X |
| [22] |
Yim, W.-L.; Liu, Z.-F. J. Am. Chem. Soc. 2001, 123, 2243.
pmid: 11456870 |
| [23] |
Kohno, Y.; Takahashi, O.; Saito, K. Phys. Chem. Chem. Phys. 2001, 3, 2742.
doi: 10.1039/b101745o |
| [24] |
Xiao, H.-M.; Ju, X.-H.; Xu, L.-N.; Fang, J.-Y. J. Chem. Phys. 2004, 121, 12523.
doi: 10.1063/1.1812258 |
| [25] |
Türker, L.; Atalar, T. J. Hazard. Mater. 2006, A137, 1333.
|
| [26] |
Xu, L.; Fang, G.; Li, X.; Yuan, J.; Hu, X.; Zhu, W.; Xiao, H.; Ji, G. J. Mol. Graphics Modell. 2007, 26, 415.
doi: 10.1016/j.jmgm.2007.01.009 |
| [27] |
Hiyoshi, R. I.; Kohno, Y.; Nakamura, J. J. Phys. Chem. A 2004, 108, 5915.
doi: 10.1021/jp049118i |
| [28] |
Hiyoshi, R. I.; Kohno, Y.; Takahashi, O.; Nakamura, J.; Yamaguchi, Y.; Matsumoto, S.; Azuma, N.; Ueda, K. J. Phys. Chem. A 2006, 110, 9816.
pmid: 16898682 |
| [29] |
Keshavarz, M. H.; Zohari, N.; Seyedsadjadi, S. A. J. Therm. Anal. Calorim. 2013, 114, 497.
doi: 10.1007/s10973-013-3022-6 |
| [30] |
Liu, Z.; Wu, Q.; Zhu, W.; Xiao, H. Phys. Chem. Chem. Phys. 2015, 17, 10568.
doi: 10.1039/C5CP00637F |
| [31] |
Moxnes, J. F.; Frøyland, Ø.; Risdal, T. J. Mol. Model. 2017, 23, 240.
doi: 10.1007/s00894-017-3408-7 pmid: 28744746 |
| [32] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
|
| [33] |
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
doi: 10.1063/1.464913 |
| [34] |
Szabo, A.; Ostlund, N. S. Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory, Ed.: Mineola, N. Y., Dover, 1996, pp. 221-229.
|
| [35] |
Grafenstein, J.; Hjerpe, A. M.; Kraka, E. J. Phys. Chem. A 2000, 104, 1748.
doi: 10.1021/jp993122q |
| [36] |
Yao, Z.; Yu, Z. J. Am. Chem. Soc. 2011, 133, 10864.
doi: 10.1021/ja2021476 |
| [37] |
Ling, L.; Liu, K.; Li, X.; Li, Y. ACS Catal. 2015, 5, 2458.
doi: 10.1021/cs501892s |
| [1] | 李光磊, 黄海丰, 杨军, 段红珍. 吡唑含能离子盐的合成研究进展[J]. 有机化学, 2021, 41(4): 1466-1488. |
| [2] | 翟连杰, 张俊林, 张家荣, 吴敏杰, 毕福强, 王伯周. N—F键调控的高能量密度化合物合成与性能研究进展[J]. 有机化学, 2020, 40(6): 1484-1501. |
| [3] | 绳利丽, 单自兴, 郭晓燕, 杨荣杰. 十二氢十二硼酸双(二烷基-5-氨基四唑)盐的合成、表征及热性能[J]. 有机化学, 2018, 38(8): 2093-2100. |
| [4] | 刘跃佳, 张晓娟, 宁弘历, 杨海君. 咪唑类含能离子液体的合成及性能研究[J]. 有机化学, 2016, 36(5): 1133-1142. |
| [5] | 代玲玲, 崔胜峰, Guri L. V. Damu, 周成合. 四唑类化合物的合成及应用研究新进展[J]. 有机化学, 2013, 33(02): 224-244. |
| [6] | 何佳, 金波, 彭汝芳, 楚士晋, 董海山. 1,1-二(2,4,6-三硝基苯甲酰胺基)-2,2-二硝基乙烯的合成[J]. 有机化学, 2011, 31(10): 1643-1647. |
| [7] | 李永祥, 王建龙, 王艳红, 曹端林, 李建东, 于艳蓉. 一种合成2-硝亚胺基-5-硝基-六氢化-1,3,5-三嗪(NNHT)的新方法[J]. 有机化学, 2011, 31(02): 256-259. |
| [8] | 王艳红; 宋元军; 胡 桢 ; 景介辉; 孟祥丽; 黄玉东*. 一个简易的2,6-二氨基-3,5-二硝基吡啶-1-氧化物的合成方法[J]. 有机化学, 2009, 29(05): 780-783. |
| [9] | 李洪珍*; 李金山 ; 黄 明 ; 周小清. 氨基呋咱氧化为氨基硝基呋咱的合成研究[J]. 有机化学, 2009, 29(05): 798-801. |
| [10] | 李洪珍*; 周小清; 李金山; 黄 明. 3-氨基-4-硝基呋咱和3,3’-二硝基-4,4’-偶氮呋咱的合成研究[J]. 有机化学, 2008, 28(9): 1646-1648. |
| [11] | 王伯周,来蔚鹏,刘愆,廉鹏,薛永强. 3,6-双(1H-1,2,3,4-四唑-5-氨基)-1,2,4,5-四嗪的合成、表征及量子化学研究[J]. 有机化学, 2008, 28(03): 422-427. |
| [12] | 李海波,程碧波,李洪珍,聂福德李金山,黄忠,刘世俊. 2,6-二氨基-3,5-二硝基吡嗪-1-氧化物的合成[J]. 有机化学, 2007, 27(01): 112-115. |
| [13] | 苗艳玲, 张同来, 乔小晶, 张建国, 郁开北. 4,6-二硝基苯并氧化呋咱的制备、晶体结构及热分解机理[J]. 有机化学, 2004, 24(2): 205-209. |
| [14] | 李战雄,唐松青,刘金涛. 3,4—二氨基呋咱的乙酰化反应[J]. 有机化学, 2002, 22(11): 902-904. |
| [15] | 阎红,邢光建,周丽丽,黄军英. 立方烷和高立方烷类衍生物的合成及应用[J]. 有机化学, 2000, 20(5): 649-654. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||