有机化学 ›› 2023, Vol. 43 ›› Issue (6): 2217-2225.DOI: 10.6023/cjoc202210027 上一篇 下一篇
研究论文
收稿日期:2022-10-24
修回日期:2022-11-28
发布日期:2022-12-21
作者简介:基金资助:
Rongbin Cai, Bing Li, Qi Zhou, Longyi Zhu*( ), Jun Luo*(
), Jun Luo*( )
)
Received:2022-10-24
Revised:2022-11-28
Published:2022-12-21
Contact:
E-mail: About author:Supported by:文章分享

小分子法多步合成结构刚性的、多官能化的氮杂金刚烷骨架一直是金刚烷化学领域的一大挑战. 本工作以双环[3.3.1]壬烷-2,6-二酮为原料, 经缩酮化、溴代、消除、Prilezhaev氧化得到双环氧化物中间体, 并在120 ℃下氨解关环合成9,10-二羟基-2-氮杂金刚烷-4,8-二酮双(乙二醇缩酮), 后经N,O-硝化合成了9,10-二硝酰氧基-2-硝基-2-氮杂金刚烷- 4,8-二酮双(乙二醇缩酮), 六步反应总收率10%; 而该双环氧化物中间体在135 ℃下氨解得到2-氮杂原金刚烷(2-氮杂三环[4.3.1.03,8]-癸烷)的骨架异构体4,10-二羟基-2-氮杂原金刚烷-5,9-二酮双(乙二醇缩酮), 后经N-乙酰化、硝化-去缩酮化得到相应的O-硝化及N,O-硝化衍生物. 为后续多官能化的2-氮杂金刚烷及其骨架异构体2-氮杂原金刚烷的衍生提供了可靠的方法, 可用作高能量密度笼型化合物的结构框架.
蔡荣斌, 李冰, 周琪, 朱隆懿, 罗军. 4,8,9,10-四官能化的2-氮杂金刚烷及其2-氮杂原金刚烷骨架异构体的合成[J]. 有机化学, 2023, 43(6): 2217-2225.
Rongbin Cai, Bing Li, Qi Zhou, Longyi Zhu, Jun Luo. Synthesis of 4,8,9,10-Tetrafunctionalized 2-Azaadamantanes and Their 2-Azaprotoadamantane Skeleton Isomers[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2217-2225.

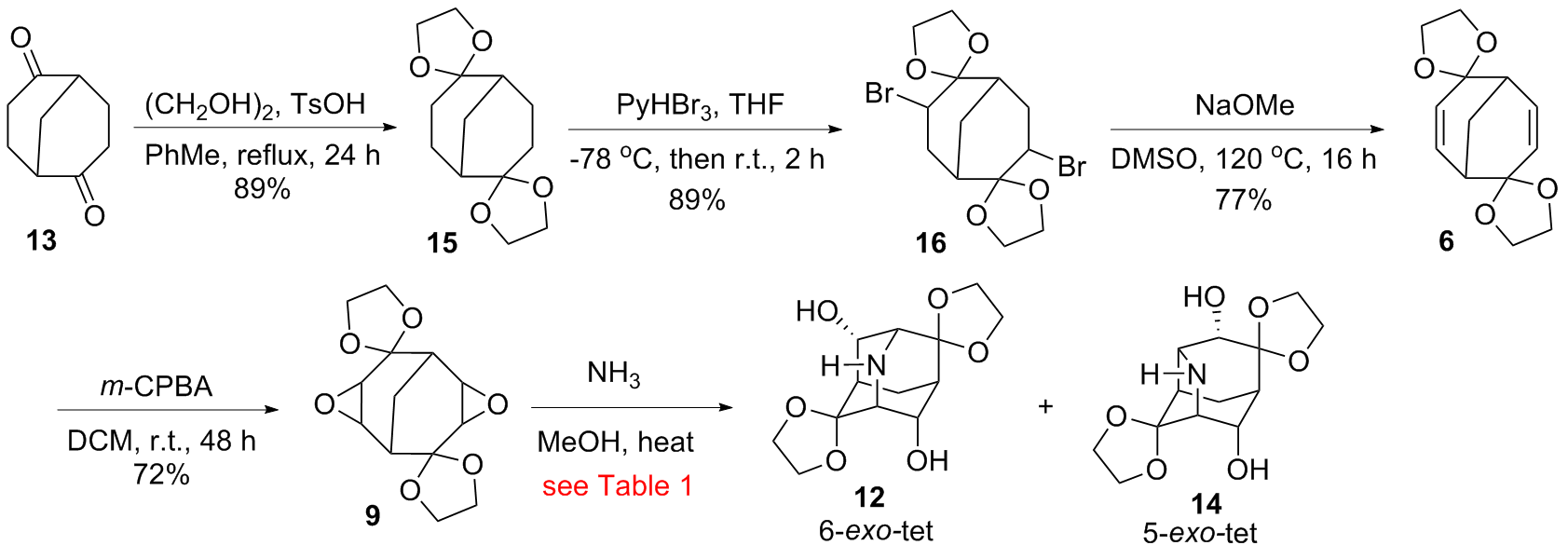
| Entry | Temp./℃ | Time/h | Recoveryb/% of 9 | Yieldb/% of 12/14 |
|---|---|---|---|---|
| 1 | 100 | 24 | 86 | <5/0 |
| 2 | 105 | 24 | 81 | 8/0 |
| 3 | 110 | 24 | 73 | 12/0 |
| 4 | 115 | 24 | 64 | 21/0 |
| 5 | 120 | 24 | 53 | 30/0 |
| 6 | 125 | 24 | 50 | 26/7 |
| 7 | 130 | 24 | 46 | 19/18 |
| 8 | 135 | 24 | 40 | 14/26 |
| 9 | 140 | 24 | 39 | 12/24 |
| 10 | 120 | 36 | 36 | 37/0 |
| 11 | 120 | 48 | 30 | 42/0 |
| 12 | 120 | 60 | 25 | 40/0 |
| 13 | 135 | 36 | 36 | 18/33 |
| 14 | 135 | 48 | 27 | 17/39 |
| 15 | 135 | 60 | 18 | 11/36 |
| 16c | 120 | 48 | 34 | 38/0 |
| 17c | 135 | 48 | 28 | 13/36 |
| 18d | 135 | 48 | — | 96/0 |
| Entry | Temp./℃ | Time/h | Recoveryb/% of 9 | Yieldb/% of 12/14 |
|---|---|---|---|---|
| 1 | 100 | 24 | 86 | <5/0 |
| 2 | 105 | 24 | 81 | 8/0 |
| 3 | 110 | 24 | 73 | 12/0 |
| 4 | 115 | 24 | 64 | 21/0 |
| 5 | 120 | 24 | 53 | 30/0 |
| 6 | 125 | 24 | 50 | 26/7 |
| 7 | 130 | 24 | 46 | 19/18 |
| 8 | 135 | 24 | 40 | 14/26 |
| 9 | 140 | 24 | 39 | 12/24 |
| 10 | 120 | 36 | 36 | 37/0 |
| 11 | 120 | 48 | 30 | 42/0 |
| 12 | 120 | 60 | 25 | 40/0 |
| 13 | 135 | 36 | 36 | 18/33 |
| 14 | 135 | 48 | 27 | 17/39 |
| 15 | 135 | 60 | 18 | 11/36 |
| 16c | 120 | 48 | 34 | 38/0 |
| 17c | 135 | 48 | 28 | 13/36 |
| 18d | 135 | 48 | — | 96/0 |
| [1] |
Guo, L. D.; Chen, Y.; Xu, J. Acc. Chem. Res. 2020, 53, 2726.
doi: 10.1021/acs.accounts.0c00532 |
| [2] |
Guo, L. D.; Hou, J.; Tu, W.; Zhang, Y.; Zhang, Y.; Chen, L.; Xu, J. J. Am. Chem. Soc. 2019, 141, 11713.
doi: 10.1021/jacs.9b05641 |
| [3] |
Piemontesi, C.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 6556.
doi: 10.1002/anie.201602374 |
| [4] |
Parchinsky, V.; Shumsky, A.; Krasavin, M. Tetrahedron Lett. 2011, 52, 7161.
doi: 10.1016/j.tetlet.2011.10.125 |
| [5] |
Ponomarev, K.; Morozova, E.; Pavlova, A.; Suslov, E.; Korchagina, D.; Nefedov, A.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Med Chem. 2017, 13, 773.
doi: 10.1021/jm00298a057 |
| [6] |
Ponomarev, K.; Pavlova, A.; Suslov, E.; Ardashov, O.; Korchagina, D.; Nefedov, A.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Med. Chem. Res. 2015, 24, 4146.
doi: 10.1007/s00044-015-1464-z |
| [7] |
Tanner, J. A.; Zheng, B. J.; Zhou, J.; Watt, R. M.; Jiang, J. Q.; Wong, K. L.; Lin, Y. P.; Lu, L. Y.; He, M. L.; Kung, H. F.; Kesel, A. J.; Huang, J. D. Chem. Biol. 2005, 12, 303.
doi: 10.1016/j.chembiol.2005.01.006 |
| [8] |
Zubairov, M. M.; Selyaninov, Y. O.; Egorova, I. Y.; Roshchin, A. V.; Kuznetsov, A. I.; Kholstov, A. V.; Tikhonov, I. P. Russ. J. Phys. Chem. B 2015, 9, 471.
doi: 10.1134/S1990793115030240 |
| [9] |
Arutyunyan, G. L.; Arutyunyan, A. D.; Gevorkyan, K. A.; Gasparyan, S. P.; Paronikyan, R. V.; Stepanyan, G. M.; Minasyan, N. S. Pharm. Chem. J. 2018, 52, 419.
doi: 10.1007/s11094-018-1834-1 |
| [10] |
Harutyunyan, G. L.; Harutyunyan, A. D.; Gevorkyan, K. A.; Paronikyan, R. V.; Stepanyan, G. M.; Gasparyan, S. P. Pharm. Chem. J. 2021, 54, 1205.
doi: 10.1007/s11094-021-02344-w |
| [11] |
Narayan, S.; Ramisetti, S.; Jaiswal, A. S.; Law, B. K.; Singh-Pillay, A.; Singh, P.; Amin, S.; Sharma, A. K. Eur. J. Med. Chem. 2019, 161, 456.
doi: S0223-5234(18)30922-X pmid: 30384048 |
| [12] |
Darout, E.; Robinson, R. P.; McClure, K. F.; Corbett, M.; Li, B.; Shavnya, A.; Andrews, M. P.; Jones, C. S.; Li, Q.; Minich, M. L.; Mascitti, V.; Guimaraes, C. R. W.; Munchhof, M. J.; Bahnck, K. B.; Cai, C.; Price, D. A.; Liras, S.; Bonin, P. D.; Cornelius, P.; Wang, R.; Bagdasarian, V.; Sobota, C. P.; Hornby, S.; Masterson, V. M.; Joseph, R. M.; Kalgutkar, A. S.; Chen, Y. J. Med. Chem. 2013, 56, 301.
doi: 10.1021/jm301626p pmid: 23234271 |
| [13] |
Xiang, D.; Chen, H.; Zhu, W.; Xiao, H. Can. J. Chem. 2016, 94, 667.
doi: 10.1139/cjc-2016-0174 |
| [14] |
Hou, T.; Ruan, H.; Wang, G.; Luo, J. Eur. J. Org. Chem. 2017, 2017, 6957.
doi: 10.1002/ejoc.v2017.46 |
| [15] |
Dupeyre, R. M.; Rassat, A. Tetrahedron Lett. 1975, 16, 1839.
doi: 10.1016/S0040-4039(00)75270-1 |
| [16] |
Furukawa, K.; Inada, H.; Shibuya, M.; Yamamoto, Y. Org. Lett. 2016, 18, 4230.
doi: 10.1021/acs.orglett.6b01964 pmid: 27533283 |
| [17] |
Rafiee, M.; Miles, K. C.; Stahl, S. S. J. Am. Chem. Soc. 2015, 137, 14751.
doi: 10.1021/jacs.5b09672 |
| [18] |
Sasano, Y.; Nagasawa, S.; Yamazaki, M.; Shibuya, M.; Park, J.; Iwabuchi, Y. Angew. Chem., Int. Ed. 2014, 53, 3236.
doi: 10.1002/anie.201309634 |
| [19] |
Shibuya, M.; Nagasawa, S.; Osada, Y.; Iwabuchi, Y. J. Org. Chem. 2014, 79, 10256.
doi: 10.1021/jo501862k |
| [20] |
Tomizawa, M.; Shibuya, M.; Iwabuchi, Y. Org. Lett. 2009, 11, 1829.
doi: 10.1021/ol900441f pmid: 19323487 |
| [21] |
Tomizawa, M.; Shibuya, M.; Iwabuchi, Y. Org. Lett. 2014, 16, 4968.
doi: 10.1021/ol502543r |
| [22] |
Uesugi, S.; Watanabe, T.; Imaizumi, T.; Ota, Y.; Yoshida, K.; Ebisu, H.; Chinen, T.; Nagumo, Y.; Shibuya, M.; Kanoh, N.; Usui, T.; Iwabuchi, Y. J. Org. Chem. 2015, 80, 12333.
doi: 10.1021/acs.joc.5b02256 |
| [23] |
Kleinlein, C.; Bendelsmith, A. J.; Zheng, S. L.; Betley, T. A. Angew. Chem., Int. Ed. 2017, 56, 12197.
doi: 10.1002/anie.v56.40 |
| [24] |
Premuzic, D.; Holynska, M.; Ozarowski, A.; Pietzonka, C.; Roseborough, A.; Stoian, S. A. Inorg. Chem. 2020, 59, 10768.
doi: 10.1021/acs.inorgchem.0c01242 |
| [25] |
Sieste, S.; Lifincev, I.; Stein, N.; Wagner, G. Dalton Trans. 2017, 46, 12226.
doi: 10.1039/C7DT02406A |
| [26] |
Black, R. M. Synthesis 1981, 1981, 829.
doi: 10.1055/s-1981-29617 |
| [27] |
Becker, D. P.; Flynn, D. L. Synthesis 1992, 1992, 1080.
doi: 10.1055/s-1992-26307 |
| [28] |
Udding, J. H.; Papin, N.; Hiemstra, H.; Speckamp, W. N. Tetrahedron 1994, 50, 8853.
doi: 10.1016/S0040-4020(01)85358-8 |
| [29] |
Gurskii, M. E.; Kolomnikova, G. D.; Baranin, S. V.; Bubnov, Y. N. Mendeleev Commun. 2018, 28, 366.
doi: 10.1016/j.mencom.2018.07.008 |
| [30] |
Risch, N. Chem. Ber. 1985, 118, 4073.
doi: 10.1002/cber.v118:10 |
| [31] |
Risch, N.; Langhals, M.; Mikosch, W.; Boegge, H.; Mueller, A. J. Am. Chem. Soc. 1991, 113, 9411.
doi: 10.1021/ja00024a081 |
| [32] |
Delpech, B.; Khuong, H. Q. J. Org. Chem. 1978, 43, 4898.
doi: 10.1021/jo00419a048 |
| [33] |
Taheri, A.; Quinn, R. J.; Krasavin, M. Tetrahedron Lett. 2014, 55, 5390.
doi: 10.1016/j.tetlet.2014.08.020 |
| [34] |
Banister, S. D.; Yoo, D. T.; Chua, S. W.; Cui, J.; Mach, R. H.; Kassiou, M. Bioorg. Med. Chem. Lett. 2011, 21, 5289.
doi: 10.1016/j.bmcl.2011.07.028 pmid: 21788137 |
| [35] |
Becker, D. P.; Flynn, D. L.; Shone, R. L.; Gullikson, G. Bioorg. Med. Chem. Lett. 2004, 14, 5509.
doi: 10.1016/j.bmcl.2004.09.005 |
| [36] |
Wu, J.; Leas, D. A.; Dong, Y.; Wang, X.; Ezell, E. L.; Stack, D. E.; Vennerstrom, J. L. ACS Omega 2018, 3, 11362.
doi: 10.1021/acsomega.8b01819 |
| [37] |
Ruan, H.; Ling, Y.; Wang, G.; Luo, J. Chin. J. Energ. Mater. 2016, 24, 544. (in Chinese)
|
|
(阮宏伟, 凌亦飞, 王桂香, 罗军, 含能材料, 2016, 24, 544.)
|
|
| [38] |
Henkel, J. G.; Faith, W. C.; Hane, J. T. J. Org. Chem. 1981, 46, 3483.
doi: 10.1021/jo00330a020 |
| [39] |
Li, G.; Nelsen, S. F.; Jalilov, A. S.; Guzei, I. A. J. Org. Chem. 2010, 75, 2445.
doi: 10.1021/jo100294u |
| [40] |
Zhang, J.; Hou, T.; Zhang, L.; Luo, J. Org. Lett. 2018, 20, 7172.
doi: 10.1021/acs.orglett.8b03107 pmid: 30394097 |
| [41] |
Ivachtchenko, A. V.; Khvat, A.; Tkachenko, S. E.; Sandulenko, Y. B.; Vvedensky, V. Y. Tetrahedron Lett. 2004, 45, 6733.
doi: 10.1016/j.tetlet.2004.07.056 |
| [42] |
Vatsadze, S. Z.; Tyurin, V. S.; Zatsman, A. I.; Manaenkova, M. A.; Semashko, V. S.; Krut'ko, D. P.; Zyk, N. V.; Churakov, A. V.; Kuz'mina, L. G. Russ. J. Org. Chem. 2006, 42, 1225.
doi: 10.1134/S1070428006080215 |
| [43] |
Arutyunyan, G. L.; Dzhagatspanyan, I. A.; Nazaryan, I. M.; Akopyan, A. G.; Arutyunyan, A. D. Pharm. Chem. J. 2007, 41, 591.
doi: 10.1007/s11094-008-0025-x |
| [44] |
Kuznetsov, A. I.; Senan, I. M.; Razenko, I. O.; Serova, T. M. Russ. Chem. Bull. 2014, 63, 2689.
doi: 10.1007/s11172-014-0800-7 |
| [45] |
Stetter, H.; Theise, D.; Steffens, G. J. Chem. Ber. 1970, 103, 200.
doi: 10.1002/cber.v103:1 |
| [46] |
Stetter, H.; Bremen, J. Chem. Ber. 1973, 106, 2523.
doi: 10.1002/cber.v106:8 |
| [47] |
Nielsen, A. T.; Chafin, A. P.; Christian, S. L.; Moore, D. W.; Nadler, M. P.; Nissan, R. A.; Vanderah, D. J.; Gilardi, R. D.; George, C. F.; Flippen-Anderson, J. L. Tetrahedron 1998, 54, 11793.
doi: 10.1016/S0040-4020(98)83040-8 |
| [48] |
Quast, H.; Berneth, C. P. Chem. Ber. 1983, 116, 1345.
doi: 10.1002/cber.v116:4 |
| [49] |
Hou, T.; Zhang, J.; Wang, C.; Luo, J. Org. Chem. Front. 2017, 4, 1819.
doi: 10.1039/C7QO00357A |
| [50] |
Semakin, A. N.; Nelyubina, Y. V.; Ioffe, S. L.; Sukhorukov, A. Y. Eur. J. Org. Chem. 2020, 2020, 6723.
doi: 10.1002/ejoc.v2020.43 |
| [51] |
Butlerow, A. Ann. Chem. Pharm. 1859, 111, 242.
doi: 10.1002/(ISSN)1099-0690 |
| [52] |
Semakin, A. N.; Sukhorukov, A. Y.; Lesiv, A. V.; Ioffe, S. L.; Lyssenko, K. A.; Nelyubina, Y. V.; Tartakovsky, V. A. Org. Lett. 2009, 11, 4072.
doi: 10.1021/ol9015157 pmid: 19739685 |
| [53] |
Semakin, A. N.; Sukhorukov, A. Y.; Nelyubina, Y. V.; Khomutova, Y. A.; Ioffe, S. L.; Tartakovsky, V. A. J. Org. Chem. 2014, 79, 6079.
doi: 10.1021/jo5007703 pmid: 24911065 |
| [54] |
Cai, R.; Zhou, Q.; Hou, T.; Li, B.; Liu, Y.; Li, H.; Gao, Y.; Zhu, L.; Luo, J. Org. Chem. Front. 2022, 9, 3684.
doi: 10.1039/D2QO00427E |
| [55] |
Klimochkin, Y. N.; Leonova, M.; Ivleva, E. Russ. J. Org. Chem. 2020, 56, 1702.
doi: 10.1134/S107042802010005X |
| [56] |
Ling, Y.; Zhang, P.; Sun, L.; Lai, W.; luo, J. Synthesis 2014, 46, 2225.
doi: 10.1055/s-00000084 |
| [57] |
Shi, Q.; Javorskis, T.; Bergquist, K.-E.; Ulcinas, A.; Niaura, G.; Matulaitiene, I.; Orentas, E.; Waernmark, K. Nat. Commun. 2017, 8, 14943.
doi: 10.1038/ncomms14943 |
| [58] |
Wallentin, C.-J.; Orentas, E.; Johnson, M. T.; Bathori, N. B.; Butkus, E.; Wendt, O. F.; Waernmark, K.; Oehrstroem, L. CrystEngComm 2012, 14, 178.
doi: 10.1039/C1CE05673E |
| [59] |
Beckmann, E.; Bahr, N.; Cullmann, O.; Yang, F.; Kegel, M.; Voegtle, M.; Exner, K.; Keller, M.; Knothe, L.; Prinzbach, H. Eur. J. Org. Chem. 2003, 2003, 4248.
doi: 10.1002/(ISSN)1099-0690 |
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [3] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [4] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [5] | 樊思捷, 董武恒, 梁彩云, 王贵超, 袁瑶, 尹作栋, 张兆国. 可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物[J]. 有机化学, 2023, 43(9): 3277-3286. |
| [6] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [7] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [8] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [9] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [10] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [11] | 马佳敏, 李姣兄, 孟千森, 曾祥华. 炔烃的自由基砜基化反应研究进展[J]. 有机化学, 2023, 43(6): 2040-2052. |
| [12] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [13] | 孔德亮, 戴闻, 赵怡玲, 陈艺林, 朱红平. 脒基胺硼基硅宾与单酮和二酮的氧化环加成反应研究[J]. 有机化学, 2023, 43(5): 1843-1851. |
| [14] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [15] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||