有机化学 ›› 2023, Vol. 43 ›› Issue (10): 3590-3597.DOI: 10.6023/cjoc202305019 上一篇 下一篇
所属专题: 有机硅化学专辑-2023
研究论文
收稿日期:2023-05-15
修回日期:2023-08-05
发布日期:2023-09-08
基金资助:
Yiling Zhao, Zhikang Chen, Lei Li, Conglei Liu, Hongping Zhu( )
)
Received:2023-05-15
Revised:2023-08-05
Published:2023-09-08
Contact:
*E-mail: Supported by:文章分享

研究了硅宾/有机铝组成的Lewis酸碱对(LP)体系引发丙烯酸酯类单体聚合的性能, 合成了4种硅宾: L(Ph2P)Si (Si-1, L=PhC(NtBu)2)、L[4-MeC6H4(Ph2P)N]Si (Si-2)、L[2,6-iPr2C6H3(1,5-C8H14B)N]Si (Si-3)、L(LSi)Si (Si-4)和6种有机铝: Al(C6F5)3、AlMe(BHT)2、AliBu(BHT)2、AliBu2(BHT)、AliBu(BHT*)2、AliBu2(BHT*) (BHT=2,6-tBu2-4-MeC6H2O, BHT*=2,4,6-tBu3C6H2O). Si-1/有机铝和Si-4/有机铝LP体系均能引发甲基丙烯酸甲酯(MMA)完全聚合, 活性转换频率(TOF)值为50~400 h-1, 产出PMMA的分子量Mn 82000~271000和分子量分布Đ 1.11~1.81. Si-2/有机铝和Si-3/有机铝LP体系没有活性. Si-1/Al(C6F5)3体系对丙烯酸正丁酯(nBA)、丙烯酸-2-乙基己基酯(EHA)的活性较差, 但是Si-1/其它有机铝都能高效引发nBA和EHA完全聚合. 对nBA, TOF值4.8×104~14.4×104 h-1, 产出PnBA的Mn 100000~179000和Đ 1.08~1.59; 对EHA, TOF值至14.4×104~36.0×104 h-1, 生成PEHA的Mn 81000~240000以及Đ 1.12~1.95. 研究了聚合反应的可能机理.
赵怡玲, 陈志康, 李磊, 刘聪磊, 朱红平. 硅宾/有机铝的Lewis酸碱对体系及其丙烯酸酯聚合的引发性能[J]. 有机化学, 2023, 43(10): 3590-3597.
Yiling Zhao, Zhikang Chen, Lei Li, Conglei Liu, Hongping Zhu. Silylene/Organoaluminum Lewis Pair System and the Initiation Property for Polymerization of (Meth)acrylates[J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3590-3597.
| Entry | LB | LA | M | M/LA/LB | t/h | Mnb | Ðb | I* c/% |
|---|---|---|---|---|---|---|---|---|
| 1 | Si-1 | Al(C6F5)3 | MMA | 200/2/1 | 3.0 | 82000 | 1.33 | 24.4 |
| 2 | Si-1 | AlMe(BHT)2 | MMA | 200/2/1 | 1.0 | 152000 | 1.47 | 13.2 |
| 3 | Si-1 | AliBu(BHT)2 | MMA | 200/2/1 | 4.0 | 122000 | 1.31 | 16.4 |
| 4 | Si-1 | AliBu2(BHT) | MMA | 200/2/1 | 0.5 | 107000 | 1.68 | 18.7 |
| 5 | Si-1 | AliBu(BHT*)2 | MMA | 200/2/1 | 3.0 | 174000 | 1.11 | 11.5 |
| 6 | Si-1 | AliBu2(BHT*) | MMA | 200/2/1 | 2.0 | 124000 | 1.66 | 16.1 |
| 7 | Si-4 | Al(C6F5)3 | MMA | 200/2/1 | 2.0 | 85000 | 1.81 | 23.5 |
| 8 | Si-4 | AlMe(BHT)2 | MMA | 200/2/1 | 3.0 | 138000 | 1.77 | 14.5 |
| 9 | Si-4 | AliBu(BHT)2 | MMA | 200/2/1 | 3.0 | 134000 | 1.56 | 14.9 |
| 10 | Si-4 | AliBu2(BHT) | MMA | 200/2/1 | 2.0 | 174000 | 1.67 | 11.5 |
| 11 | Si-4 | AliBu(BHT*)2 | MMA | 200/2/1 | 3.0 | 202000 | 1.74 | 9.9 |
| 12 | Si-4 | AliBu2(BHT*) | MMA | 200/2/1 | 3.0 | 271000 | 1.31 | 7.4 |
| Entry | LB | LA | M | M/LA/LB | t/h | Mnb | Ðb | I* c/% |
|---|---|---|---|---|---|---|---|---|
| 1 | Si-1 | Al(C6F5)3 | MMA | 200/2/1 | 3.0 | 82000 | 1.33 | 24.4 |
| 2 | Si-1 | AlMe(BHT)2 | MMA | 200/2/1 | 1.0 | 152000 | 1.47 | 13.2 |
| 3 | Si-1 | AliBu(BHT)2 | MMA | 200/2/1 | 4.0 | 122000 | 1.31 | 16.4 |
| 4 | Si-1 | AliBu2(BHT) | MMA | 200/2/1 | 0.5 | 107000 | 1.68 | 18.7 |
| 5 | Si-1 | AliBu(BHT*)2 | MMA | 200/2/1 | 3.0 | 174000 | 1.11 | 11.5 |
| 6 | Si-1 | AliBu2(BHT*) | MMA | 200/2/1 | 2.0 | 124000 | 1.66 | 16.1 |
| 7 | Si-4 | Al(C6F5)3 | MMA | 200/2/1 | 2.0 | 85000 | 1.81 | 23.5 |
| 8 | Si-4 | AlMe(BHT)2 | MMA | 200/2/1 | 3.0 | 138000 | 1.77 | 14.5 |
| 9 | Si-4 | AliBu(BHT)2 | MMA | 200/2/1 | 3.0 | 134000 | 1.56 | 14.9 |
| 10 | Si-4 | AliBu2(BHT) | MMA | 200/2/1 | 2.0 | 174000 | 1.67 | 11.5 |
| 11 | Si-4 | AliBu(BHT*)2 | MMA | 200/2/1 | 3.0 | 202000 | 1.74 | 9.9 |
| 12 | Si-4 | AliBu2(BHT*) | MMA | 200/2/1 | 3.0 | 271000 | 1.31 | 7.4 |
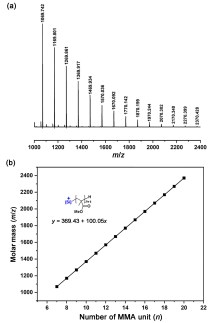

| Entry | LA | M | t/s | Conv.b/% | Mnc | Ðc | I*d/% |
|---|---|---|---|---|---|---|---|
| 1 | Al(C6F5)3 | nBA | 60 | 50 | — | — | — |
| 2 | AlMe(BHT)2 | nBA | 5 | >99 | 113000 | 1.08 | 17.7 |
| 3 | AliBu(BHT)2 | nBA | 10 | >99 | 115000 | 1.31 | 17.4 |
| 4 | AliBu2(BHT) | nBA | 15 | >99 | 130000 | 1.54 | 15.4 |
| 5 | AliBu(BHT*)2 | nBA | 10 | >99 | 179000 | 1.36 | 11.2 |
| 6 | AliBu2(BHT*) | nBA | 5 | >99 | 100000 | 1.59 | 20.0 |
| 7 | Al(C6F5)3 | EHA | 60 | 66.7 | — | — | — |
| 8 | AlMe(BHT)2 | EHA | 2 | >99 | 156000 | 1.10 | 12.8 |
| 9 | AliBu(BHT)2 | EHA | 5 | >99 | 157000 | 1.12 | 12.7 |
| 10 | AliBu2(BHT) | EHA | 2 | >99 | 240000 | 1.52 | 8.3 |
| 11 | AliBu(BHT*)2 | EHA | 5 | >99 | 120000 | 1.66 | 16.7 |
| 12 | AliBu2(BHT*) | EHA | 5 | >99 | 224000 | 1.63 | 8.9 |
| Entry | LA | M | t/s | Conv.b/% | Mnc | Ðc | I*d/% |
|---|---|---|---|---|---|---|---|
| 1 | Al(C6F5)3 | nBA | 60 | 50 | — | — | — |
| 2 | AlMe(BHT)2 | nBA | 5 | >99 | 113000 | 1.08 | 17.7 |
| 3 | AliBu(BHT)2 | nBA | 10 | >99 | 115000 | 1.31 | 17.4 |
| 4 | AliBu2(BHT) | nBA | 15 | >99 | 130000 | 1.54 | 15.4 |
| 5 | AliBu(BHT*)2 | nBA | 10 | >99 | 179000 | 1.36 | 11.2 |
| 6 | AliBu2(BHT*) | nBA | 5 | >99 | 100000 | 1.59 | 20.0 |
| 7 | Al(C6F5)3 | EHA | 60 | 66.7 | — | — | — |
| 8 | AlMe(BHT)2 | EHA | 2 | >99 | 156000 | 1.10 | 12.8 |
| 9 | AliBu(BHT)2 | EHA | 5 | >99 | 157000 | 1.12 | 12.7 |
| 10 | AliBu2(BHT) | EHA | 2 | >99 | 240000 | 1.52 | 8.3 |
| 11 | AliBu(BHT*)2 | EHA | 5 | >99 | 120000 | 1.66 | 16.7 |
| 12 | AliBu2(BHT*) | EHA | 5 | >99 | 224000 | 1.63 | 8.9 |
| [1] |
Baskaran D. Prog. Polym. Sci. 2003, 28, 521.
doi: 10.1016/S0079-6700(02)00083-7 |
| [2] |
Kwon Y.; Faust R. Adv. Polym. Sci. 2004, 167, 107.
|
| [3] |
Kanazawa A.; Kanaoka S.; Aoshima S. Chem. Lett. 2010, 39, 1232.
doi: 10.1246/cl.2010.1232 |
| [4] |
Webster O. W.; Hertler W. R.; Sogah D. Y.; Farnham W. B.; Rajanbabu T. V. J. Am. Chem. Soc. 1983, 105, 5706.
doi: 10.1021/ja00355a039 |
| [5] |
Webster O. W. J. Polym. Sci., Part A 2000, 38, 2855.
doi: 10.1002/(ISSN)1099-0518 |
| [6] |
Anastasaki A.; Nikolaou V.; Nurumbetov G.; Wilson P.; Kempe K.; Quinn J. F.; Davis T. P.; Whittaker M. R.; Haddleton D. M. Chem. Rev. 2016, 116, 835.
doi: 10.1021/acs.chemrev.5b00191 pmid: 26226544 |
| [7] |
Ouchi M.; Sawamoto M. Macromolecules 2017, 50, 2603.
doi: 10.1021/acs.macromol.6b02711 |
| [8] |
Xiao L.; Zhang Y.; Hong M. Chin. J. Org. Chem. 2023, 43, 949 (in Chinese).
doi: 10.6023/cjoc202301009 |
|
(肖丽娟, 张艳平, 洪缪, 有机化学, 2023, 43, 949.)
doi: 10.6023/cjoc202301009 |
|
| [9] |
Wang Q.; Zhao W.; Zhang S.; He J.; Zhang Y.; Chen E. Y.-X. ACS Catal. 2018, 8, 3571.
doi: 10.1021/acscatal.8b00333 |
| [10] |
Guan Y.; Chang K.; Sun Q.; Xu X. Chin. J. Org. Chem. 2022, 42, 1326 (in Chinese).
doi: 10.6023/cjoc202112008 |
|
(管怡雯, 常克俭, 孙千林, 徐信, 有机化学, 2022, 42, 1326.)
doi: 10.6023/cjoc202112008 |
|
| [11] |
Zhao W.; Wang Q.; He J.; Zhang Y. Polym. Chem. 2019, 10, 4328.
doi: 10.1039/C9PY00626E |
| [12] |
Zhang P.; Zhou H.; Lu X. Macromolecules 2019, 52, 4520.
doi: 10.1021/acs.macromol.9b00652 |
| [13] |
Wang X.; Hong M. Macromolecules 2020, 53, 4659.
doi: 10.1021/acs.macromol.0c00553 |
| [14] |
Zhang Z.; Wang X.; Wang X.; Li Y.; Hong M. Macromolecules 2021, 54, 8495.
doi: 10.1021/acs.macromol.1c01356 |
| [15] |
Jia Y.; Ren W.; Liu S.; Xu T.; Wang Y.; Lu X. ACS Macro. Lett. 2014, 3, 896.
doi: 10.1021/mz500437y |
| [16] |
Ottou W. N.; Conde-Mendizabal E.; Pascual A.; Wirotius A. L.; Bourichon D.; Vignolle J.; Robert F.; Landais Y.; Sotiropoulos J. M.; Miqueu K.; Taton D. Macromolecules 2017, 50, 762.
doi: 10.1021/acs.macromol.6b02205 |
| [17] |
Xu P.; Xu X. ACS Catal. 2018, 8, 198.
doi: 10.1021/acscatal.7b02875 |
| [18] |
Kikuchi S.; Chen Y.; Kitano K.; Sato S.-I.; Satoh T.; Kakuchi T. Macromolecules 2016, 49, 3049.
doi: 10.1021/acs.macromol.6b00190 |
| [19] |
Zhao W.; He J.; Zhang Y. Sci. Bull. 2019, 64, 1830.
doi: 10.1016/j.scib.2019.08.025 |
| [20] |
Hong M.; Chen J.; Chen E. Y.-X. Chem. Rev. 2018, 118, 10551.
doi: 10.1021/acs.chemrev.8b00352 |
| [21] |
McGraw M. L.; Chen E. Y.-X. Macromolecules 2020, 53, 6102.
doi: 10.1021/acs.macromol.0c01156 |
| [22] |
Zhang Y.; Miyake G. M.; John M. G.; Falivene L.; Caporaso L.; Cavallo L.; Chen E. Y.-X. Dalton Trans. 2012, 41, 9119.
doi: 10.1039/c2dt30427a |
| [23] |
Jia Y.; Wang Y.; Ren W.; Xu T.; Wang J.; Lu X. Macromolecules 2014, 47, 1966.
doi: 10.1021/ma500047d |
| [24] |
Wang Q. Y.; Zhao W. C.; He J. H.; Zhang Y. T.; Chen E. Y.-X. Macromolecules 2017, 50, 123.
doi: 10.1021/acs.macromol.6b02398 |
| [25] |
Chen Y.; Shen J.; Liu S.; Zhao J.; Wang Y.; Zhang G. Macromolecules 2018, 51, 8286.
doi: 10.1021/acs.macromol.8b01852 |
| [26] |
Walther P.; Krauß A.; Naumann S. Angew. Chem., Int. Ed. 2019, 58, 10737.
doi: 10.1002/anie.v58.31 |
| [27] |
Murahashi S.; Nozakura S.; Hatada K.; Takeuchi S.; Aoki T. Seniken Nenpo 1960, 13, 99.
|
| [28] |
Ikeda M.; Hirano T.; Tsuruta T. Makromol. Chem. 1971, 150, 127.
doi: 10.1002/macp.02.v150:1 |
| [29] |
Kitayama T.; Iijima T.; Nishiura T.; Hatada K. Polym. Bull. 1992, 28, 327.
doi: 10.1007/BF00294830 |
| [30] |
Zhang Y.; Miyake G. M.; Chen E. Y.-X. Angew. Chem., Int. Ed. 2010, 49, 10158.
doi: 10.1002/anie.v49.52 |
| [31] |
Wang X.; Zhang Y.; Hong M. Molecules 2018, 23, 442.
doi: 10.3390/molecules23020442 |
| [32] |
Zhou Y.; Jiang S.; Xu X. Chin. J. Chem. 2021, 39, 149.
doi: 10.1002/cjoc.v39.1 |
| [33] |
Yao S.-L.; Xiong Y.; Driess M. Organometallics 2011, 30, 1748.
doi: 10.1021/om200017h |
| [34] |
Hadlington T. J.; Driess M.; Jones C. Chem. Soc. Rev. 2018, 47, 4176.
doi: 10.1039/c7cs00649g pmid: 29666847 |
| [35] |
Shan C.; Yao S.; Driess M. Chem. Soc. Rev. 2020, 49, 6733.
doi: 10.1039/D0CS00815J |
| [36] |
Vidal F.; Lin H.; Morales C.; Jäkle F. Molecules 2018, 23, 405.
doi: 10.3390/molecules23020405 |
| [37] |
Chia C.-C.; Li Y.; Xiao L.; Yang M.-C.; Su M.-D.; So C.-W. Eur. J. Org. Chem. 2022, e202200003.
|
| [38] |
Hadlington T. J.; Driess M.; Jones C. Chem. Soc. Rev. 2018, 47, 4176.
doi: 10.1039/c7cs00649g pmid: 29666847 |
| [39] |
Shan C.; Yao S.; Driess M. Chem. Soc. Rev. 2020, 49, 6733.
doi: 10.1039/D0CS00815J |
| [40] |
Zhao Y.; Chen Y.; Zhang L.; Li J.; Peng Y.; Chen Z.; Jiang L.; Zhu H. Inorg. Chem. 2022, 61, 5215.
doi: 10.1021/acs.inorgchem.1c03349 |
| [41] |
Chen Y.; Li J.; Zhao Y.; Zhang L.; Tan G.; Zhu H.; Roesky H. W. J. Am. Chem. Soc. 2021, 143, 2212.
doi: 10.1021/jacs.0c12908 |
| [42] |
Li J.; Goffitzer D. J.; Xiang M.; Chen Y.; Jiang W.; Diefenbach M.; Zhu H.; Holthausen M. C.; Roesky H. W. J. Am. Chem. Soc. 2021, 143, 8244.
doi: 10.1021/jacs.1c03149 |
| [43] |
Chen Y.; Chen Z.; Jiang L; Li J.; Zhao Y.; Zhu H.; Roesky H. W. Chem. Eur. J. 2022, 28, e2021037.
|
| [44] |
Kong D.; Dai W.; Zhao Y.; Chen Y.; Zhu H. Chin. J. Org. Chem. 2023, 43, 1843 (in Chinese).
doi: 10.6023/cjoc202212006 |
|
(孔德亮, 戴闻, 赵怡玲, 陈艺林, 朱红平, 有机化学, 2023, 43, 1843.)
doi: 10.6023/cjoc202212006 |
|
| [45] |
Chen Z.; Zhao W.; Liu C.; Jiang L.; Fu G. ; Zhang Y.; Zhu H. Polym. Chem. 2023, 14, 2344.
doi: 10.1039/D3PY00301A |
| [46] |
Azhakar R.; Ghadwal R. S.; Roesky H. W.; Wolf H.; Stalke D. Organometallics 2012, 31, 4588.
doi: 10.1021/om3003762 |
| [47] |
Sen S. S.; Jana A.; Roesky H. W.; Schulzke C. Angew. Chem., Int. Ed. 2009, 48, 8536.
doi: 10.1002/anie.v48:45 |
| [48] |
So C. W.; Roesky H. W.; Magull J.; Oswald R. B. Angew. Chem., Int. Ed. 2006, 45, 3948.
|
| [49] |
Sen S. S.; Roesky H. W.; Stern D.; Henn J.; Stalke D. J. Am. Chem. Soc. 2010, 132, 1123.
doi: 10.1021/ja9091374 |
| [50] |
Chen J.; Chen E. Y. X. Dalton Trans. 2016, 45, 6105.
doi: 10.1039/C5DT03895B |
| [51] |
Lee C. H.; Lee S. J.; Park J. W.; Kim K. H.; Lee B. Y.; Oh J. S. J. Mol. Catal. A-Chem. 1998, 132, 231.
|
| [52] |
Stapleton R. A.; Al-Humydi A.; Chai J.; Galan B. R.; Collins S. Organometallics 2006, 25, 5083.
doi: 10.1021/om060474s |
| [53] |
Faingol’d E. E.; Bravaya N. M.; Panin A. N.; Babkina O. N.; Saratovskikh S. L.; Privalov V. I. J. Appl. Polym. Sci. 2016, 133, 43276.
|
| [54] |
He J.; Zhang Y.; Falivene L.; Caporaso L.; Cavallo L.; Chen E. Y.-X. Macromolecules 2014, 47, 7765.
doi: 10.1021/ma5019389 |
| [55] |
Bai Y.; Hea J.; Zhang Y. Angew. Chem., Int. Ed. 2018, 57, 17230.
doi: 10.1002/anie.v57.52 |
| [56] |
Bai Y.; Wang H.; He J.; Zhang Y. Polym. Chem. 2021, 12, 5548.
doi: 10.1039/D1PY00924A |
| [57] |
Bai Y.; Wang H.; He J.; Zhang Y.; Chen E. Y.-X. Nat. Commun. 2021, 12, 4874.
doi: 10.1038/s41467-021-25069-6 |
| [58] |
Ge F.; Li S.; Wang Z.; Zhang W.; Wang X. Polym. Chem. 2021, 12, 4226.
doi: 10.1039/D1PY00579K |
| [59] |
Khan S.; Pal S.; Kathewad N.; Purushothaman I.; De S.; Parameswaran P. Chem. Commun. 2016, 52, 3880.
doi: 10.1039/C6CC00597G |
| [60] |
Bin Ismail M. L.; So C.-W. Chem. Commun. 2019, 55, 2074.
doi: 10.1039/C8CC09454C |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||