有机化学 ›› 2025, Vol. 45 ›› Issue (1): 22-41.DOI: 10.6023/cjoc202406040 上一篇 下一篇
综述与进展
方家恒a,b, 田润妍b, 陈继君b,*( ), 刘心元b,*(
), 刘心元b,*( )
)
收稿日期:2024-06-27
修回日期:2024-07-10
发布日期:2024-07-25
基金资助:
Jiaheng Fanga,b, Runyan Tianb, Jijun Chenb( ), Xinyuan Liub(
), Xinyuan Liub( )
)
Received:2024-06-27
Revised:2024-07-10
Published:2024-07-25
Contact:
*E-mail: Supported by:文章分享

α-叔胺是一类在氮原子α位具有叔碳中心的胺类化合物. 由于叔碳中心的存在, α-叔胺在生物体内的脂溶性与代谢稳定性有所改变, 使其在药物研发领域具有重要的应用价值. 传统的α-叔胺合成方法包括: 酮亚胺的亲核加成、羰基化合物的亲电胺化、三级烯丙基亲电试剂的烯丙基胺化及重排反应等. 得益于自由基具有高活性、反应条件温和及良好的官能团兼容性, 自由基反应在合成α-叔胺方面表现出独特优势. 近年来, 发展出了烯烃的自由基胺化/氢胺烷基化、自由基碳氮交叉偶联、亚胺的自由基加成及烷烃的碳氢键活化等方法. 综述了近十年来自由基介导的分子间α-叔胺的合成方法, 并详细讨论了每种方法的特点.
方家恒, 田润妍, 陈继君, 刘心元. 自由基介导的α-叔胺合成研究进展[J]. 有机化学, 2025, 45(1): 22-41.
Jiaheng Fang, Runyan Tian, Jijun Chen, Xinyuan Liu. Research Progress in the Synthesis of α-Tertiary Amines via Radical Strategies[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 22-41.
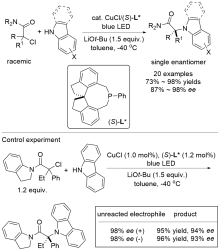
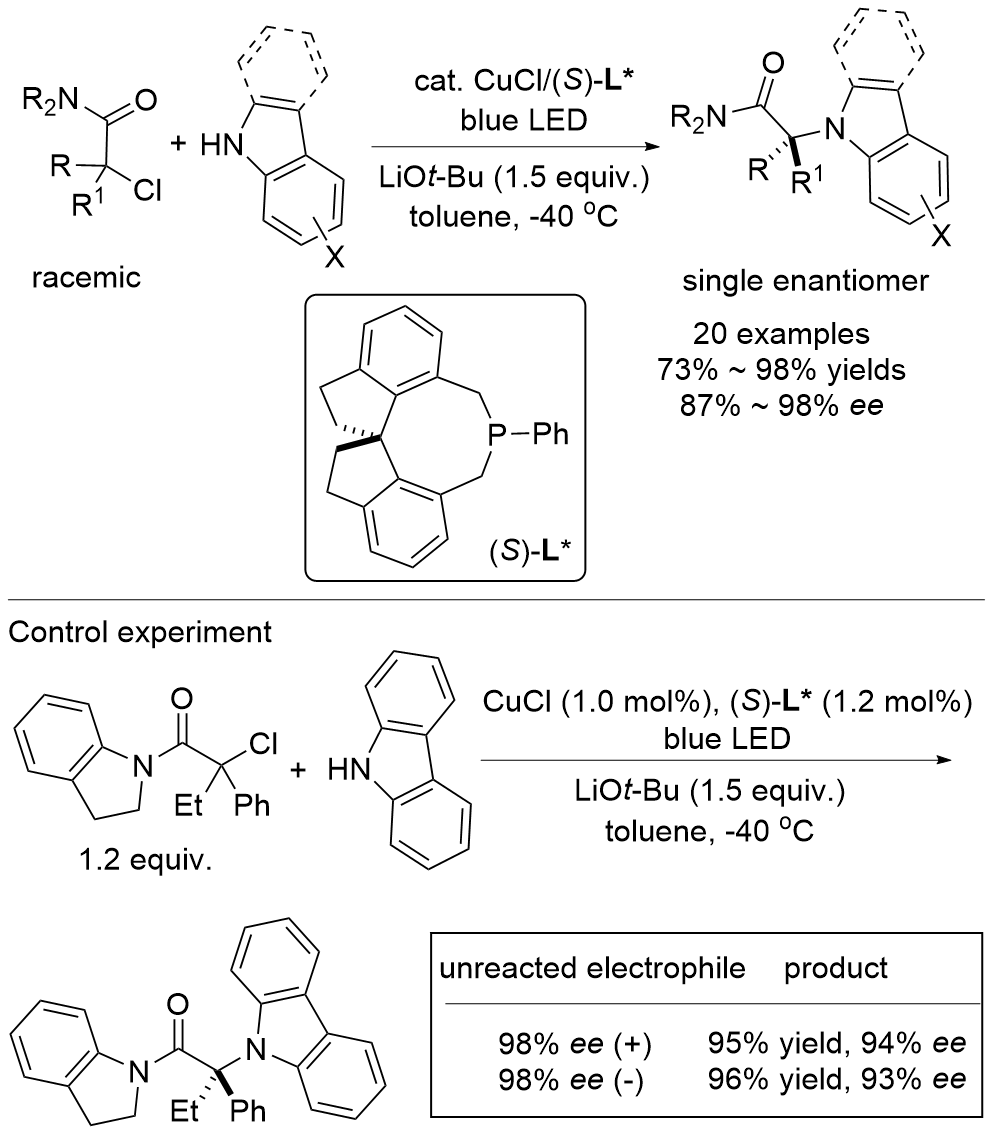
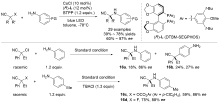

| [1] |
Hager, A.; Vrielink, N.; Hager, D.; Lefranc, J.; Trauner, D. Nat. Prod. Rep. 2016, 33, 491.
|
| [2] |
Wanka, L.; Iqbal, K.; Schreiner, P. R. Chem. Rev. 2013, 113, 3516.
|
| [3] |
Ramos-Gonzalez, N.; Paul, B.; Majumdar, S. Pharmacol. Res. 2023, 197, 106961.
|
| [4] |
Xu, Y.; Wang, J.; Deng, G.-J.; Shao, W. Chem. Commun. 2023, 59, 4099.
|
| [5] |
Kiyokawa, K.; Watanabe, T.; Fra, L.; Kojima, T.; Minakata, S. J. Org. Chem. 2017, 82, 11711.
|
| [6] |
Nakagawa, A.; Iwai, Y.; Hashimoto, H.; Miyazaki, N.; Oiwa, R.; Takahashi, Y.; Hirano, A.; Shibukawa, N.; Kojima, Y.; Omura, S. J. Antibiot. 1981, 34, 1408.
|
| [7] |
Kimura, T.; Suga, T.; Kameoka, M.; Ueno, M.; Inahashi, Y.; Matsuo, H.; Iwatsuki, M.; Shigemura, K.; Shiomi, K.; Takahashi, Y.; Omura, S.; Nakashima, T. J. Antibiot. 2019, 72, 169.
|
| [8] |
Morgenthaler, M.; Schweizer, E.; Hoffmann-Röder, A.; Benini, F.; Martin, R. E.; Jaeschke, G.; Wagner, B.; Fischer, H.; Bendels, S.; Zimmerli, D.; Schneider, J.; Diederich, F.; Kansy, M.; Müeller, K. ChemMedChem 2007, 2, 1100.
|
| [9] |
Shibasaki, M.; Kanai, M. Chem. Rev. 2008, 108, 2853.
|
| [10] |
Enders, D.; Gottfried, K.; Raabe, G. Adv. Synth. Catal. 2010, 352, 3147.
|
| [11] |
Bloch, R. Chem. Rev. 1998, 98, 1407.
|
| [12] |
Yamada, K.-I.; Tomioka, K. Chem. Rev. 2008, 108, 2874.
|
| [13] |
Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626.
|
| [14] |
Curto, J. M.; Dickstein, J. S.; Berritt, S.; Kozlowski, M. C. Org. Lett. 2014, 16, 1948.
|
| [15] |
Wieland, L. C.; Vieira, E. M.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2008, 131, 570.
|
| [16] |
Trost, B. M.; Tracy, J. S.; Lin, E. Y. ACS Catal. 2019, 9, 11082.
|
| [17] |
Ohmatsu, K.; Ando, Y.; Nakashima, T.; Ooi, T. Chem 2016, 1, 802.
|
| [18] |
Guo, W.; Cai, A.; Xie, J.; Kleij, A. W. Angew. Chem., Int. Ed. 2017, 56, 11797.
|
| [19] |
Cai, A.; Guo, W.; Martínez-Rodríguez, L.; Kleij, A. W. J. Am. Chem. Soc. 2016, 138, 14194.
|
| [20] |
Arnold, J. S.; Nguyen, H. M. J. Am. Chem. Soc. 2012, 134, 8380.
|
| [21] |
Clayden, J.; Donnard, M.; Lefranc, J.; Tetlow, D. J. Chem. Commun. 2011, 47, 4624.
|
| [22] |
Iosub, V.; Haberl, A. R.; Leung, J.; Tang, M.; Vembaiyan, K.; Parvez, M.; Back, T. G. J. Org. Chem. 2010, 75, 1612.
|
| [23] |
Arnold, J. S.; Cizio, G. T.; Nguyen, H. M. Org. Lett. 2011, 13, 5576.
|
| [24] |
Romero, K. J.; Galliher, M. S.; Pratt, D. A.; Stephenson, C. R. J. Chem. Soc. Rev. 2018, 47, 7851.
|
| [25] |
Yan, M.; Lo, J. C.; Edwards, J. T.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 12692.
|
| [26] |
Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. Acc. Chem. Res. 2020, 53, 170.
|
| [27] |
Sibi, M. P.; Manyem, S.; Zimmerman, J. Chem. Rev. 2003, 103, 3263.
|
| [28] |
Bunescu, A.; Ha, T. M.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2017, 56, 10555.
|
| [29] |
Bao, X.; Yokoe, T.; Ha, T. M.; Wang, Q.; Zhu, J. Nat. Commun. 2018, 9, 3725.
|
| [30] |
Forster, D.; Guo, W.; Wang, Q.; Zhu, J. ACS Catal. 2021, 11, 10871.
|
| [31] |
Carlson, A. S.; Topczewski, J. J. Org. Biomol. Chem. 2019, 17, 4406.
|
| [32] |
Lv, D.; Sun, Q.; Zhou, H.; Ge, L.; Qu, Y.; Li, T.; Ma, X.; Li, Y.; Bao, H. Angew. Chem., Int. Ed. 2021, 60, 12455.
|
| [33] |
Liu, W.; Pu, M.; He, J.; Zhang, T.; Dong, S.; Liu, X.; Wu, Y.-D.; Feng, X. J. Am. Chem. Soc. 2021, 143, 11856.
|
| [34] |
Ge, L.; Wang, H.; Liu, Y.; Feng, X. J. Am. Chem. Soc. 2024, 146, 13347.
|
| [35] |
Wu, L.; Zhang, Z.; Wu, D.; Wang, F.; Chen, P.; Lin, Z.; Liu, G. Angew. Chem., Int. Ed. 2021, 60, 6997.
|
| [36] |
Suh, S.-E.; Chen, S.-J.; Mandal, M.; Guzei, I. A.; Cramer, C. J.; Stahl, S. S. J. Am. Chem. Soc. 2020, 11388.
|
| [37] |
Gockel, S. N.; Buchanan, T. L.; Hull, K. L. J. Am. Chem. Soc. 2018, 140, 58.
|
| [38] |
Nicely, A. M.; Popov, A. G.; Wendlandt, H. C.; Trammel, G. L.; Kohler, D. G.; Hull, K. L. Org. Lett. 2023, 25, 5302.
|
| [39] |
Gui, J.; Pan, C.-M.; Jin, Y.; Qin, T.; Lo, J. C.; Lee, B. J.; Spergel, S. H.; Mertzman, M. E.; Pitts, W. J.; La Cruz, T. E.; Schmidt, M. A.; Darvatkar, N.; Natarajan, S. R.; Baran, P. S. Science 2015, 348, 886.
|
| [40] |
Zhu, K.; Shaver, M. P.; Thomas, S. P. Chem. Sci. 2016, 7, 3031.
|
| [41] |
Trowbridge, A.; Reich, D.; Gaunt, M. J. Nature 2018, 561, 522.
|
| [42] |
Henry Blackwell, J.; Harris, G. R.; Smith, M. A.; Gaunt, M. J. J. Am. Chem. Soc. 2021, 143, 15946.
|
| [43] |
Harris, G. R.; Trowbridge, A. D.; Gaunt, M. J. Org. Lett. 2023, 25, 861.
|
| [44] |
Ashley, M. A.; Yamauchi, C.; Chu, J. C. K.; Otsuka, S.; Yorimitsu, H.; Rovis, T. Angew. Chem., Int. Ed. 2019, 58, 4002.
|
| [45] |
Ye, J.; Kalvet, I.; Schoenebeck, F.; Rovis, T. Nat. Chem. 2018, 10, 1037.
|
| [46] |
Ryder, A. S. H.; Cunningham, W. B.; Ballantyne, G.; Mules, T.; Kinsella, A. G.; Turner-Dore, J.; Alder, C. M.; Edwards, L. J.; McKay, B. S. J.; Grayson, M. N.; Cresswell, A. J. Angew. Chem., Int. Ed. 2020, 59, 14986.
|
| [47] |
Askey, H. E.; Grayson, J. D.; Tibbetts, J. D.; Turner-Dore, J. C.; Holmes, J. M.; Kociok-Kohn, G.; Wrigley, G. L.; Cresswell, A. J. J. Am. Chem. Soc. 2021, 143, 15936.
|
| [48] |
Liu, W.-Q.; Lee, B. C.; Song, N.; He, Z.; Shen, Z.-A.; Lu, Y.; Koh, M. J. Angew. Chem., Int. Ed. 2024, 63, e202402140.
|
| [49] |
Fisher, D. J.; Burnett, G. L.; Velasco, R.; Read de Alaniz, J. J. Am. Chem. Soc. 2015, 137, 11614.
|
| [50] |
Janey, J. M. Angew. Chem., Int. Ed. 2005, 44, 4292.
|
| [51] |
Kainz, Q. M.; Matier, C. D.; Bartoszewicz, A.; Zultanski, S. L.; Peters, J. C.; Fu, G. C. Science 2016, 351, 681.
|
| [52] |
Peacock, D. M.; Roos, C. B.; Hartwig, J. F. ACS Cent. Sci. 2016, 2, 647.
|
| [53] |
Cho, H.; Suematsu, H.; Oyala, P. H.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2022, 144, 4550.
|
| [54] |
Zhang, Y.-F.; Wang, J.-H.; Yang, N.-Y.; Chen, Z.; Wang, L.-L.; Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. Angew. Chem., Int. Ed. 2023, 62, e202302983.
|
| [55] |
Chen, J.-J.; Zhang, J.-Y.; Fang, J.-H.; Du, X.-Y.; Xia, H.-D.; Cheng, B.; Li, N.; Yu, Z.-L.; Bian, J.-Q.; Wang, F.-L.; Zheng, J.-J.; Liu, W.-L.; Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. J. Am. Chem. Soc. 2023, 145, 14686.
|
| [56] |
Zheng, J.-J.; Liu, W.-L.; Gu, Q.-S.; Li, Z.-L.; Chen, J.-J.; Liu, X.-Y. Precis. Chem. 2023, 1, 576.
|
| [57] |
Chen, J.-J.; Fang, J.-H.; Du, X.-Y.; Zhang, J.-Y.; Bian, J.-Q.; Wang, F.-L.; Luan, C.; Liu, W.-L.; Liu, J.-R.; Dong, X.-Y.; Li, Z.-L.; Gu, Q.-S.; Dong, Z.; Liu, X.-Y. Nature 2023, 618, 294.
|
| [58] |
Du, X.-Y.; Fang, J.-H.; Chen, J.-J.; Shen, B.; Liu, W.-L.; Zhang, J.-Y.; Ye, X.-M.; Yang, N.-Y.; Gu, Q.-S.; Li, Z.-L.; Yu, P.; Liu, X.-Y. J. Am. Chem. Soc. 2024, 146, 9444.
|
| [59] |
Gong, Y.; Zhu, Z.; Qian, Q.; Tong, W.; Gong, H. Org. Lett. 2021, 23, 1005.
|
| [60] |
Duan, G.; Qian, Q.; Chen, Y. Tetrahedron Lett. 2023, 129, 154730.
|
| [61] |
Jeffrey, J. L.; Petronijević, F. R.; MacMillan, D. W. C. J. Am. Chem. Soc. 2015, 137, 8404.
|
| [62] |
Brueckner, A. C.; Hancock, E. N.; Anders, E. J.; Tierney, M. M.; Morgan, H. R.; Scott, K. A.; Lamar, A. A. Org. Biomol. Chem. 2016, 14, 4387.
|
| [63] |
Zhang, H.-H.; Yu, S. J. Org. Chem. 2017, 82, 9995.
|
| [64] |
Rong, J. W.; Seeberger, P. H.; Gilmore, K. Org. Lett. 2018, 20, 4081.
|
| [65] |
Nicastri, M. C.; Lehnherr, D.; Lam, Y.-H.; DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2020, 142, 987.
|
| [66] |
Li, S.; Du, H.-W.; Davies, P. W.; Shu, W. CCS Chem. 2024, 6, 1060.
|
| [67] |
Tomono, R.; Kawasaki, T.; Ishida, N.; Murakami, M. Chem. Lett. 2021, 50, 1972.
|
| [68] |
Zhao, H.; Hu, Y.; Zheng, S.; Yuan, W. Org. Lett. 2023, 25, 6699.
|
| [69] |
Dickstein, J. S.; Kozlowski, M. C. Chem. Soc. Rev. 2008, 37, 1166.
|
| [70] |
Lamas, M.-C.; Vaillard, S. E.; Wibbeling, B.; Studer, A. Org. Lett. 2010, 12, 2072.
|
| [71] |
Li, Y.; Zhou, K.; Wen, Z.; Cao, S.; Shen, X.; Lei, M.; Gong, L. J. Am. Chem. Soc. 2018, 140, 15850.
|
| [72] |
Li, Y.; Lei, M.; Gong, L. Nat. Catal. 2019, 2, 1016.
|
| [73] |
Blackwell, J. H.; Kumar, R.; Gaunt, M. J. J. Am. Chem. Soc. 2021, 143, 1598.
|
| [74] |
Phelps, J. M.; Kumar, R.; Robinson, J. D.; Chu, J. C. K.; Flodén, N. J.; Beaton, S.; Gaunt, M. J. J. Am. Chem. Soc. 2024, 146, 9045.
|
| [75] |
Song, X.; Zhang, Y.; Li, Y.; Zhao, X.; Yin, Y.; Ban, X.; Jiang, Z. ACS Catal. 2023, 13, 6396.
|
| [76] |
Wu, X.; Xia, H.; Gao, C.; Luan, B.; Wu, L.; Zhang, C.; Yang, D.; Hou, L.; Liu, N.; Xia, T.; Li, H.; Qu, J.; Chen, Y. Nat. Chem. 2023, 16, 398.
|
| [77] |
Xia, T.; Wu, Y.; Hu, J.; Wu, X.; Qu, J.; Chen, Y. Angew. Chem., Int. Ed. 2024, 63, e202316012.
|
| [78] |
Xia, T.; Wu, W.; Wu, X.; Qu, J.; Chen, Y. Angew. Chem., Int. Ed. 2024, 63, e202318991.
|
| [79] |
Sharma, A.; Hartwig, J. F. Nature 2015, 517, 600.
|
| [80] |
Niu, L.; Jiang, C.; Liang, Y.; Liu, D.; Bu, F.; Shi, R.; Chen, H.; Chowdhury, A. D.; Lei, A. J. Am. Chem. Soc. 2020, 142, 17693.
|
| [81] |
Hu, A.; Guo, J.-J.; Pan, H.; Tang, H.; Gao, Z.; Zuo, Z. J. Am. Chem. Soc. 2018, 140, 1612.
|
| [82] |
An, Q.; Wang, Z.; Chen, Y.; Wang, X.; Zhang, K.; Pan, H.; Liu, W.; Zuo, Z. J. Am. Chem. Soc. 2020, 142, 6216.
|
| [1] | 令天鹏, 秦海涛, 刘峰. C(sp3)—H键对映选择性自由基反应的最新进展[J]. 有机化学, 2025, 45(2): 498-515. |
| [2] | 杨俊峰, 赵艳秋, 时磊. 电子供体-受体(EDA)复合物驱动的N-α位C—H键活化[J]. 有机化学, 2025, 45(2): 559-573. |
| [3] | 刘慧英, 吴中天, 李昊天, 吴新鑫. 铜催化砜基诱导的区域选择性C(sp3)—H键杂芳基化反应[J]. 有机化学, 2025, 45(1): 297-306. |
| [4] | 张瑞, 何萌萌, 向焌钧, 蔡莎莉, 葛从伍, 高希珂. 核扩展的萘二酰亚胺-插烯四硫富瓦烯类双极性有机半导体[J]. 有机化学, 2024, 44(9): 2810-2819. |
| [5] | 王华斌, 徐连华, 刘雄伟, 潘博文, 姚震, 黄强, 周英. N-溴代丁二酰亚胺促进的P(O)-H化合物参与的醇的直接磷酸化反应[J]. 有机化学, 2024, 44(9): 2847-2853. |
| [6] | 王丽丽, 张洲, 王廷良, 王兴兰, 毛远湖, 张吉泉. t-BuOK/DMF促进的通过自由基过程实现吲哚酮的C-3位硫化反应[J]. 有机化学, 2024, 44(9): 2898-2905. |
| [7] | 陈璐怡, 谭梦霞, 金迦南, 张子彬, 黄飞鹤, 李世军, 李云霞. 手性亚胺有机分子笼的合成及应用研究[J]. 有机化学, 2024, 44(9): 2617-2639. |
| [8] | 蒋镓西, 刘全忠. 乙烯基重氮化合物非金属卡宾机制参与的反应[J]. 有机化学, 2024, 44(9): 2640-2657. |
| [9] | 张朝阳, 罗维纬, 周俊. 烯醇硅醚参与的自由基反应研究进展[J]. 有机化学, 2024, 44(9): 2658-2681. |
| [10] | 曹茜娴, 由君, 刘其业, 刘波, 喻艳超, 武文菊. (4S,4'S)-2,2'-(4,6-二苯并呋喃二基)双[4,5-二氢-4-苯基噁唑]-镍(II)配合物催化高对映选择性氰亚胺的环加成反应[J]. 有机化学, 2024, 44(7): 2315-2332. |
| [11] | 李文雅, 王煜, 陈江琦, 史丹, 张良, 余小春, 王正军. 可见光催化不对称Minisci反应研究进展[J]. 有机化学, 2024, 44(7): 2110-2123. |
| [12] | 王文贵, 王守锋. 水溶液中的Minisci反应研究进展[J]. 有机化学, 2024, 44(7): 2136-2146. |
| [13] | 陆玲依, 邱晓东. 自由基形式烯烃双烷基化反应研究进展[J]. 有机化学, 2024, 44(6): 1701-1718. |
| [14] | 李文多, 魏娜娜, 冯楠. 硼自由基促进的C—C键形成反应构筑联芳基和苄基羧酸甲酯[J]. 有机化学, 2024, 44(6): 1853-1861. |
| [15] | 高燊原, 诸昊穹, 金巧玲, 金露儿, 王晓钟, 戴立言. 三氟甲硫基自由基引发涉及烯烃、AgSCF3和喹喔啉酮的三组分反应[J]. 有机化学, 2024, 44(4): 1264-1275. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||