有机化学 ›› 2025, Vol. 45 ›› Issue (4): 1119-1136.DOI: 10.6023/cjoc202407002 上一篇 下一篇
综述与进展
收稿日期:2024-09-20
修回日期:2024-10-29
发布日期:2024-11-20
基金资助:
Yanqiu Li, Yawei Jia, Pangkuan Chen( )
)
Received:2024-09-20
Revised:2024-10-29
Published:2024-11-20
Contact:
* E-mail: Supported by:文章分享

手性π-共轭材料通常展现出优异的光物理性质以及稳定的手性特性, 在有机光电材料领域具有广泛的应用. 由于三芳基硼(Ar3B)独特的电子特性将三芳基硼骨架嵌入到手性π-共轭体系中, 使其体系展现出优异的手性光学特性, 形成手性共轭有机三芳基硼材料, 在化学和材料科学领域均引起广泛关注. 首先介绍了手性共轭有机三芳基硼材料领域的基本概念. 接着, 根据手性来源, 分别从轴手性、螺旋手性、面手性以及中心手性讨论了手性共轭三芳基硼材料的合成策略、结构特性, 并深入探讨了这些材料在吸收、发射、荧光量子效率等手性光电性质和应用方面的最新进展, 这些特性对于手性有机光电器件的性能至关重要. 最后, 对当前研究中的挑战和未来发展趋势进行了展望, 合理预期了手性共轭有机三芳基硼材料的发展潜力和应用前景.
李艳秋, 贾亚薇, 陈磅宽. 手性共轭有机三芳基硼(Ar3B)发光材料研究进展[J]. 有机化学, 2025, 45(4): 1119-1136.
Yanqiu Li, Yawei Jia, Pangkuan Chen. Recent Progress of Chiral Conjugated Organic Triarylboron (Ar3B) Luminescent Materials[J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1119-1136.

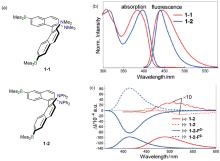

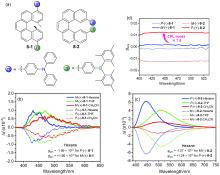
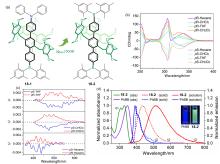
| Compound | λabs/nm | λem/nm | ΦPL/% | ǀglumǀ(×10-3) |
|---|---|---|---|---|
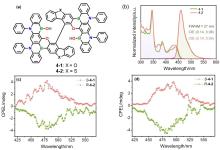
| 1-2 | 396a | 472 | 58 | 1.53 |
| 2-2 | 332b | 445 | 15 | 0.36 |
| 3-2 | 358b | 436 | 81 | 1.30 |
| 4-2 | — | 459 | 91 | — |
| Compound | λabs/nm | λem/nm | ΦPL/% | ǀglumǀ(×10-3) |
|---|---|---|---|---|
| 1-2 | 396a | 472 | 58 | 1.53 |
| 2-2 | 332b | 445 | 15 | 0.36 |
| 3-2 | 358b | 436 | 81 | 1.30 |
| 4-2 | — | 459 | 91 | — |
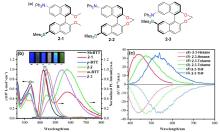




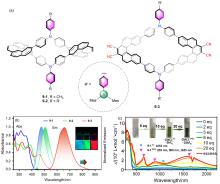
| Compound | λabs/nm | λem/nm | ΦPL/% | ǀglumǀ (×10-3) |
|---|---|---|---|---|
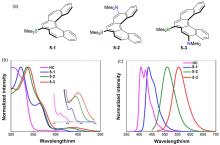
| 5-1 | 413a | 443 | 80 | — |
| 6-1 | 422b | 445 | 51 | 6.51 |
| 7-2 | 425b | 452 | 5 | 3.56 |
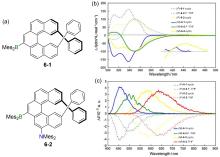
| 8-1 | 319c | 459 | 21 | 1.56 |
| 9-2 | 381a | 504 | 100 | 4.69 |
| 10-1 | 467d | 500 | 88 | — |
| 11-1 | 627a | 600 | 100 | 33 |
| 12-2 | 505d | 520 | 85 | 1.1 |
| 13-2 | 427d | 444 | 95 | 1.5 |
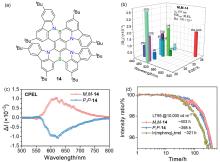
| 14 | 592 | — | — | 1.7 |
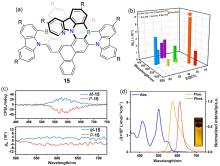
| 15 | 546d | 578 | 98 | 5.8 |
| Compound | λabs/nm | λem/nm | ΦPL/% | ǀglumǀ (×10-3) |
|---|---|---|---|---|
| 5-1 | 413a | 443 | 80 | — |
| 6-1 | 422b | 445 | 51 | 6.51 |
| 7-2 | 425b | 452 | 5 | 3.56 |
| 8-1 | 319c | 459 | 21 | 1.56 |
| 9-2 | 381a | 504 | 100 | 4.69 |
| 10-1 | 467d | 500 | 88 | — |
| 11-1 | 627a | 600 | 100 | 33 |
| 12-2 | 505d | 520 | 85 | 1.1 |
| 13-2 | 427d | 444 | 95 | 1.5 |
| 14 | 592 | — | — | 1.7 |
| 15 | 546d | 578 | 98 | 5.8 |






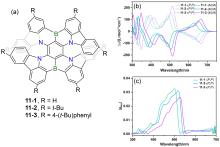






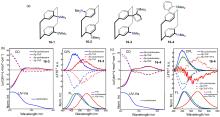

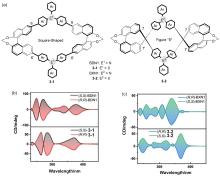
| Compound | λabs/nm | λem/nm | ΦPL/% | ǀglumǀ(×10-3) |
|---|---|---|---|---|
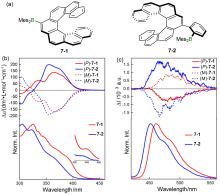
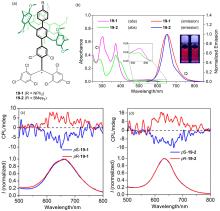
| 16-1 | 374a | 531 | 46 | 4.24 |
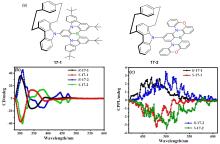
| 17-1 | — | 478 | 98 | 0.54 |
| 18-2 | 345b | 390 | 99 | 4.62 |
| 19 | 553c | 653 | 7.6 | — |
| Compound | λabs/nm | λem/nm | ΦPL/% | ǀglumǀ(×10-3) |
|---|---|---|---|---|
| 16-1 | 374a | 531 | 46 | 4.24 |
| 17-1 | — | 478 | 98 | 0.54 |
| 18-2 | 345b | 390 | 99 | 4.62 |
| 19 | 553c | 653 | 7.6 | — |





| [1] |
Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Chem. Rev. 2017, 117, 3479.
doi: 10.1021/acs.chemrev.6b00076 pmid: 27258218 |
| [2] |
Hirai, M.; Tanaka, N.; Sakai, M.; Yamaguchi, S. Chem. Rev. 2019, 119, 8291.
doi: 10.1021/acs.chemrev.8b00637 |
| [3] |
Mellerupab, S. K.; Wang, S. N. Chem. Soc. Rev. 2019, 48, 3537.
doi: 10.1039/c9cs00153k pmid: 31070642 |
| [4] |
Wang, X.; Wang, S. N. Chem. Rec. 2019, 19, 1693.
|
| [5] |
Wang, M.; Zhao, C. H. Chem. Rec. 2022, 22, e202100199.
|
| [6] |
Keerthika, P.; Konidena, R. K. Adv. Opt. Mater. 2023, 11, 2301732.
|
| [7] |
Shi, J. Q.; Ran, Z. Y.; Peng, F. W.; Chen, M. H.; Li, L.; Ji, L.; Huang, W. J. Mater. Chem. C 2022, 10, 9165.
|
| [8] |
Fu, Y. B.; Yang, H.; Gao, Y. X.; Huang, L.; Berger, R.; Liu, J. Z.; Lu, H. L.; Cheng, Z. H.; Du, S. X.; Gao, H. J.; Feng, X. L. Angew. Chem., Int. Ed. 2020, 59, 8873.
|
| [9] |
Jia, Y. W.; Li, P. F.; Liu, K. L.; Li, C. L.; Liu, M. Y.; Di, J. Q.; Wang, N.; Yin, X. D.; Zhang, N.; Chen, P. K. Chem. Sci. 2022, 13, 11672.
|
| [10] |
Zhang, Y.; Du, C. Z.; Li, J. K.; Wang, X. Y. Chin. J. Org. Chem. 2023, 43, 1645 (in Chinese).
|
|
(张祎, 杜呈卓, 李继坤, 王小野, 有机化学, 2023, 43, 1645.)
doi: 10.6023/cjoc202212037 |
|
| [11] |
Guo, J. X.; Yang, Y.; Dou, C. D.; Wang, Y. J. Am. Chem. Soc. 2021, 143, 18272.
|
| [12] |
Chen, C.; Du, C. Z.; Wang, X. Y. Adv. Sci. 2022, 9, 2200707.
|
| [13] |
Zhang, P. F.; Zeng, J. C.; Zhuang, F. D.; Zhao, K. X.; Sun, Z. H.; Yao, Z. F.; Lu, Y.; Wang, X. Y.; Wang, J. Y.; Pei, J. Angew. Chem., Int. Ed. 2021, 60, 23313.
|
| [14] |
Zhang, Y. W.; Zhang, D. D.; Huang, T. Y.; Gillett, A. J.; Liu, Y.; Hu, D. P.; Cui, L. S.; Bin, Z. Y.; Li, G. M.; Wei, J. B.; Duan, L. Angew. Chem., Int. Ed. 2021, 60, 20498.
|
| [15] |
Chen, P. K.; Marshall, A. S.; Chi, S. H.; Yin, X. D.; Perry, J. W.; Jäkle, F. Chem.-Eur. J. 2015, 21, 18237.
|
| [16] |
Meng, B.; Ren, Y.; Liu, J.; Jäkle, F.; Wang, L. X. Angew. Chem., Int. Ed. 2018, 57, 2183
|
| [17] |
Huang, Z. G.; Wang, S. N.; Dewhurst, R. D.; Ignat'ev, N. V.; Finze, M.; Braunschweig, H. Angew. Chem., Int. Ed. 2020, 59, 8800.
|
| [18] |
Li, C. L.; Shi, Y. F.; Li, P. F.; Zhang, N.; Wang, N.; Yin, X. D.; Chen, P. K. Org. Lett. 2021, 23, 7123.
|
| [19] |
Chen, P. K.; Lalancette, R. A.; Jäkle, F. Angew. Chem., Int. Ed. 2012, 51, 7994.
|
| [20] |
Xu, X. Y.; Liu, M. Y.; Li, C. L.; Liu, X. G. Chin. J. Org. Chem. 2023, 43, 1611 (in Chinese).
|
|
(徐晓阳, 刘美艳, 李成龙, 刘旭光, 有机化学, 2023, 43, 1611.)
doi: 10.6023/cjoc202212038 |
|
| [21] |
Xu, X. Y.; Liu, M. Y.; Li, C. L.; Wu, X. M.; Liu, X. G. Chin. J. Org. Chem. 2023, 43, 3826 (in Chinese).
|
|
(徐晓阳, 刘美艳, 李成龙, 吴晓明, 刘旭光, 有机化学, 2023, 43, 3826.)
doi: 10.6023/cjoc202304027 |
|
| [22] |
Chen, X.; Meng, G. Y.; Liao, G. M.; Rauch, F.; He, J.; Friedrich, A.; Marder, T.; Wang, N.; Chen, P. K.; Wang, S. N.; Yin, X. D. Chem.- Eur. J. 2021, 27, 6274.
|
| [23] |
Brandt, J. R.; Salerno, F.; Fuchter, M. J. Nat. Chem. Rev. 2017, 1, 45.
|
| [24] |
Li, Z. C.; Liu, W. W.; Cheng, H.; Chen, S. Q.; Tian, J. G. Sci. Rep. 2017, 7, 8204.
|
| [25] |
Jiang, W. G.; Pacella, M. S.; Athanasiadou, D.; Nelea, V.; Vali, H.; Hazen, R. M.; Gray, J. J.; McKee, M. D. Nat. Commun. 2017, 8, 15066.
|
| [26] |
Yao, X.; Hu, Y. W.; Cao, B.; Peng, R.; Ding, J. D. Biomaterials 2013, 34, 9001
|
| [27] |
Carr, R.; Evans, N. H.; Parker, D. Chem. Soc. Rev. 2012, 41, 7673.
|
| [28] |
Wagenknecht, C.; Li, C. M.; Reingruber, A.; Bao, X. H.; Goebel, A.; Chen, Y. A.; Zhang, Q.; Chen, K.; Pan, J. W. Nat. Photonics 2010, 4, 549.
|
| [29] |
Farshchi, R.; Ramsteiner, M.; Herfort, J.; Tahraoui, A.; Grahn, H. T. Appl. Phys. Lett. 2011, 98, 162508.
|
| [30] |
Song, F. Y.; Wei, G.; Jiang, X. X.; Li, F.; Zhu, C. J.; Cheng, Y. C. Chem. Commun. 2013, 49, 5772.
|
| [31] |
Stalder, M.; Schadt, M. Opt. Lett. 1996, 21, 1948.
pmid: 19881855 |
| [32] |
Jin, Q. X.; Chen, S. X.; Sang, Y. T.; Guo, H. Q.; Dong, S. Z.; Han, J. L.; Chen, W. J.; Yang, X. F.; Li, F.; Duan, P. F. Chem. Commun. 2019, 55, 6583.
|
| [33] |
Fan, H. H.; Li, K.; Tao Tu, T.; Zhu, X. F.; Zhang, L.; Liu, M. H. Angew. Chem., Int. Ed. 2022, 61, e202200727.
|
| [34] |
Li, P. F.; Jia, Y. W.; Zhang, S. H.; Di, J. Q.; Zhang, N.; Chen, P. K. Inorg. Chem. 2022, 61, 3951.
|
| [35] |
Sun, W. T.; Guo, J. X.; Fan, Z. M.; Yuan, L. Z.; Ye, K. Q.; Dou, C. D.; Wang, Y. Angew. Chem., Int. Ed. 2022, 61, e202209271.
|
| [36] |
Gaedke, M.; Witte, F.; Anhäuser, J.; Hupatz, H.; Schröder, H. V.; Valkonen, A.; Rissanen, K.; Lützen, A.; Paulusa, B.; Schalley, C. A. Chem. Sci. 2019, 10, 10003.
|
| [37] |
Maynard, J. R. J.; Gallagher, P.; Lozano, D.; Butler, P.; Goldup, S. M. Nat. Chem. 2022, 14, 1038.
doi: 10.1038/s41557-022-00973-6 pmid: 35760959 |
| [38] |
Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. J. Am. Chem. Soc. 2019, 141, 11852.
doi: 10.1021/jacs.9b06240 pmid: 31322357 |
| [39] |
Sun, Z. B.; Liu, J. K.; Yuan, D. F.; Zhao, Z. H.; Zhu, X. Z.; Liu, D. H.; Peng, Q.; Zhao, C. H. Angew. Chem., Int. Ed. 2019, 58, 4840.
|
| [40] |
Zhang, K.; Zhao, J.; Zhang, N.; Chen, J. F.; Wang, N.; Yin, X.; Zheng, X. Y.; Chen, P. K. J. Mater. Chem. C 2022, 10, 1816.
|
| [41] |
Zhang, K.; Hao, M. Y.; Jin, T. Y.; Shi, Y. F.; Tian, G. Q.; Li, C. L.; Ma, H. W.; Zhang, N.; Li, Q. S.; Chen, P. K. Chem.-Eur. J. 2024, 30, e202302950.
|
| [42] |
Yuan, L.; Xu, J. W.; Yan, Z. P.; Yang, Y. F.; Mao, D.; Hu, J. J.; Ni, H. X.; Li, C. H.; Zuo, J. L.; Zheng, Y. X. Angew. Chem., Int. Ed. 2024, e202407277.
|
| [43] |
Shen, Y. J.; Yao, N. T.; Diao, L. N.; Yang, Y.; Chen, X. L.; Gong, H. Y. Angew. Chem., Int. Ed. 2023, 62, e202300840.
|
| [44] |
Xiao, X.; Cheng, Q.; Bao, S. T.; Jin, Z. X.; Sun, S. T.; Jiang, H. Y.; Steigerwald, M. L.; Nuckolls, C. J. Am. Chem. Soc. 2022, 144, 20214.
|
| [45] |
Zhao, Z. H.; Zhang, M. Y.; Liu, D. H.; Zhao, C. H. Org. Lett. 2018, 20, 7590.
|
| [46] |
Zhao, Z. H.; Liang, X.; He, M. X.; Zhang, M. Y.; Zhao, C. H. Org. Lett. 2019, 21, 9569.
|
| [47] |
Li, H. W.; Li, M.; Zhao, Z. H.; Chen, C. F.; Peng, Q.; Zhao, C. H. Org. Lett. 2021, 23, 4759.
|
| [48] |
Zhao, F.; Zhao, J. Y.; Wang, Y.; Liu, H. T.; Shang, Q. H.; Wang, N.; Yin, X. D.; Zheng, X. Y.; Chen, P. K. Dalton Trans. 2022, 51, 6226.
doi: 10.1039/d2dt00677d pmid: 35362491 |
| [49] |
Zhao, F.; Zhao, J. Y.; Liu, H. T.; Wang, Y.; Duan, J. X.; Li, C. L.; Di, J. Q.; Zhang, N.; Zheng, X. Y.; Chen, P. K. J. Am. Chem. Soc. 2023, 145, 10092.
doi: 10.1021/jacs.3c00306 pmid: 37125835 |
| [50] |
Wu, X. G.; Huang, J. W.; Su, B. K.; Wang, S.; Yuan, L.; Zheng, W. Q.; Zhang, H.; Zheng, Y. X.; Zhu, W.; Chou, P. T. Adv. Mater. 2021, 34, 2105080.
|
| [51] |
Li, J. K.; Chen, X. Y.; Guo, Y. L.; Wang, X. C.; Sue, A. C. H.; Cao, X. Y.; Wang, X. Y. J. Am. Chem. Soc. 2021, 143, 17958.
|
| [52] |
Zhang, Y. W.; Zhang, D. D.; Huang, T. Y.; Gillett, A. J.; Yang Liu, Y.; Hu, D. P.; Cui, L. S.; Bin, Z. Y.; Li, G. M.; Wei, J. B.; Duan, L. Angew. Chem., Int. Ed. 2021, 60, 20498.
|
| [53] |
Zhang, F. Y.; Rauch, F.; Swain, A.; Marder, T. B.; Ravat, P. Angew. Chem., Int. Ed. 2023, 62, e202218965
|
| [54] |
Ye, Z. Y.; Wu, H.; Xu, Y. L.; Hua, T.; Chen, G. H.; Chen, Z. X.; Yin, X. J.; Huang, M. L.; Xu, K.; Song, X. F.; Huang, Z. Y.; Lv, X. L.; Miao, J. S.; Cao, X. S.; Yang, C. L. Adv. Mater. 2024, 36, 2308314.
|
| [55] |
Meng, G. Y.; Zhou, J. P.; Han, X. S.; Zhao, W. L.; Zhang, Y. W.; Li, M.; Chen, C. F.; Zhang, D. D.; Duan, L. Adv. Mater. 2024, 36, 2307420.
|
| [56] |
Guo, W. C.; Zhao, W. L.; Tan, K. K.; Li, M.; Chen, C. F. Angew. Chem., Int. Ed. 2024, 63, e202401835.
|
| [57] |
Kato, K.; Fa, S. X.; Ogoshi, T. Angew. Chem., Int. Ed. 2023, 62, e202308316.
|
| [58] |
Zhang, M. Y.; Li, Z. Y.; Lu, B.; Wang, Y.; Ma, Y. D.; Zhao, C. H. Org. Lett. 2018, 20, 6868.
|
| [59] |
Zhang, M. Y.; Li, Z. Y.; Lu, B.; Wang, Y.; Ma, Y. D.; Zhao, C. H. Org. Lett. 2021, 23, 2.
|
| [60] |
Liao, X. J.; Pu, D. D.; Yuan, L.; Tong, J. J.; Xing, S.; Tu, Z. L.; Zuo, J. L.; Zheng, W. H.; Zheng, Y. X. Angew. Chem., Int. Ed. 2023, 62, e202217045.
|
| [61] |
Chen, J. F.; Yin, X. D.; Wang, B. W.; Zhang, K.; Meng, G. Y.; Zhang, S. H.; Shi, Y. F.; Wang, N.; Wang, S. N.; Chen, P. K. Angew. Chem., Int. Ed. 2020, 59, 11267.
|
| [62] |
Chen, J. F.; Gao, Q. X.; Liu, L. J.; Chen, P. K.; Wei, T. B. Chem. Sci. 2023, 14, 987.
|
| [63] |
Chen, J. F.; Tian, G. Q.; Liu, K. L.; Zhang, N.; Wang, N.; Yin, X. D.; Chen, P. K. Org. Lett. 2022, 24, 1935.
|
| [64] |
Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Chem. Rev. 2016, 116, 13752.
pmid: 27754649 |
| [65] |
Adelizzi, B.; Chidchob, P.; Tanaka, N.; Lamers, B. A. G.; Meskers, S. C. J.; Ogi, S.; Palmans, A. R. A.; Yamaguchi, S.; Meijer, E. W. J. Am. Chem. Soc. 2020, 142, 16681.
|
| [1] | 李旻昊, 王泽溟, 黄庆, 左伟伟. 钴(II)催化的酮不对称转移氢化反应[J]. 有机化学, 2025, 45(7): 2451-2460. |
| [2] | 任天磊, 汪鑫, 丛欢. 蒽光二聚体衍生的手性单膦配体: 借助化学拆分的制备路线和不对称烯丙基胺化的合成应用[J]. 有机化学, 2025, 45(6): 2208-2221. |
| [3] | 王霜, 毛羊杰, 娄绍杰, 许丹倩. 基于氧化型导向基团的不对称C—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(6): 1961-1994. |
| [4] | 唐梦瑶, 杨晓瑜. 手性磷酸催化不对称亲电胺化反应研究进展[J]. 有机化学, 2025, 45(6): 1785-1818. |
| [5] | 朱晓宇, 杨诗林, 罗宜铭, 李文泽. 金和手性磷酸共催化的不对称合成研究进展[J]. 有机化学, 2025, 45(4): 1178-1193. |
| [6] | 林恩泽, 李必杰. 基于碳氢键断裂的金属催化的内烯烃不对称氢芳基化进展[J]. 有机化学, 2025, 45(2): 546-558. |
| [7] | 邹瑜, 郭伟聪, 汪君. 不含手性取代基的平面手性环戊二烯基铑催化剂在不对称碳氢键活化中的应用[J]. 有机化学, 2025, 45(2): 466-476. |
| [8] | 张朝威, 徐兵斌, 刘文龙, 赵敬, 段伟良. 钯催化不对称碳氢键活化合成平面手性二茂铁磺酰胺化合物[J]. 有机化学, 2025, 45(2): 707-716. |
| [9] | 袁晨晖, 焦雷. 手性配体在钯催化配位辅助对映选择性C(sp3)—H键官能团化反应中的应用[J]. 有机化学, 2025, 45(2): 602-619. |
| [10] | 陈璐怡, 谭梦霞, 金迦南, 张子彬, 黄飞鹤, 李世军, 李云霞. 手性亚胺有机分子笼的合成及应用研究[J]. 有机化学, 2024, 44(9): 2617-2639. |
| [11] | 夏羽菲, 江丽, 杨巧, 于琇, 陈丰坤. 圆偏振发光三重氮杂[6]螺烯: N-烷基化调控其手性光学性质[J]. 有机化学, 2024, 44(9): 2841-2846. |
| [12] | 王家晟, 王泽树, 何卫民, 叶龙武. 邻炔基苯胺氢胺化合成轴手性吲哚研究进展[J]. 有机化学, 2024, 44(6): 1786-1792. |
| [13] | 刘晓东, 施世良. ANIPE配体促进的铜催化联烯与亚胺和联硼试剂的不对称碳硼化反应[J]. 有机化学, 2024, 44(6): 1884-1896. |
| [14] | 李非凡, 余康, 倪传志, 朱园园, 曾婕, 古双喜. 测定氨基酸浓度和对映体组成的手性荧光探针[J]. 有机化学, 2024, 44(6): 1862-1869. |
| [15] | 刘晨光. 含氮芳香性杂环化合物的不对称氢化反应研究进展[J]. 有机化学, 2024, 44(5): 1403-1422. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||