有机化学 ›› 2025, Vol. 45 ›› Issue (2): 466-476.DOI: 10.6023/cjoc202410002 上一篇 下一篇
综述与进展
收稿日期:2024-10-05
修回日期:2024-11-24
发布日期:2024-12-26
基金资助:
Yu Zou, Weicong Guo, Jun Wang( )
)
Received:2024-10-05
Revised:2024-11-24
Published:2024-12-26
Contact:
*E-mail: wangjun23@mail.sysu.edu.cn
Supported by:文章分享

手性环戊二烯基铑(CpRh)催化剂由于具有高稳定性、可调节性和独特的反应性而被广泛应用于不对称碳氢键活化反应中, 可以将简单有机分子高效转化为具有中心手性、平面手性、轴手性和螺旋手性的分子. 根据手性CpRh催化剂中Cp配体是否具有手性, 可将催化剂分为手性Cp衍生的催化剂和前手性Cp衍生的催化剂. 目前, 前者的开发已取得巨大成功, 而后者的研究较少. 综述了不含手性取代基的平面手性环戊二烯基铑催化剂的合成及其在不对称碳氢键活化反应中的应用, 并介绍了反应机理及立体控制模型. 最后, 探讨了该领域所面临的机遇与挑战.
邹瑜, 郭伟聪, 汪君. 不含手性取代基的平面手性环戊二烯基铑催化剂在不对称碳氢键活化中的应用[J]. 有机化学, 2025, 45(2): 466-476.
Yu Zou, Weicong Guo, Jun Wang. Application of Planar Chiral Cyclopentadienyl Rhodium Catalysts without Chiral Substituents in Asymmetric C—H Activation[J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 466-476.

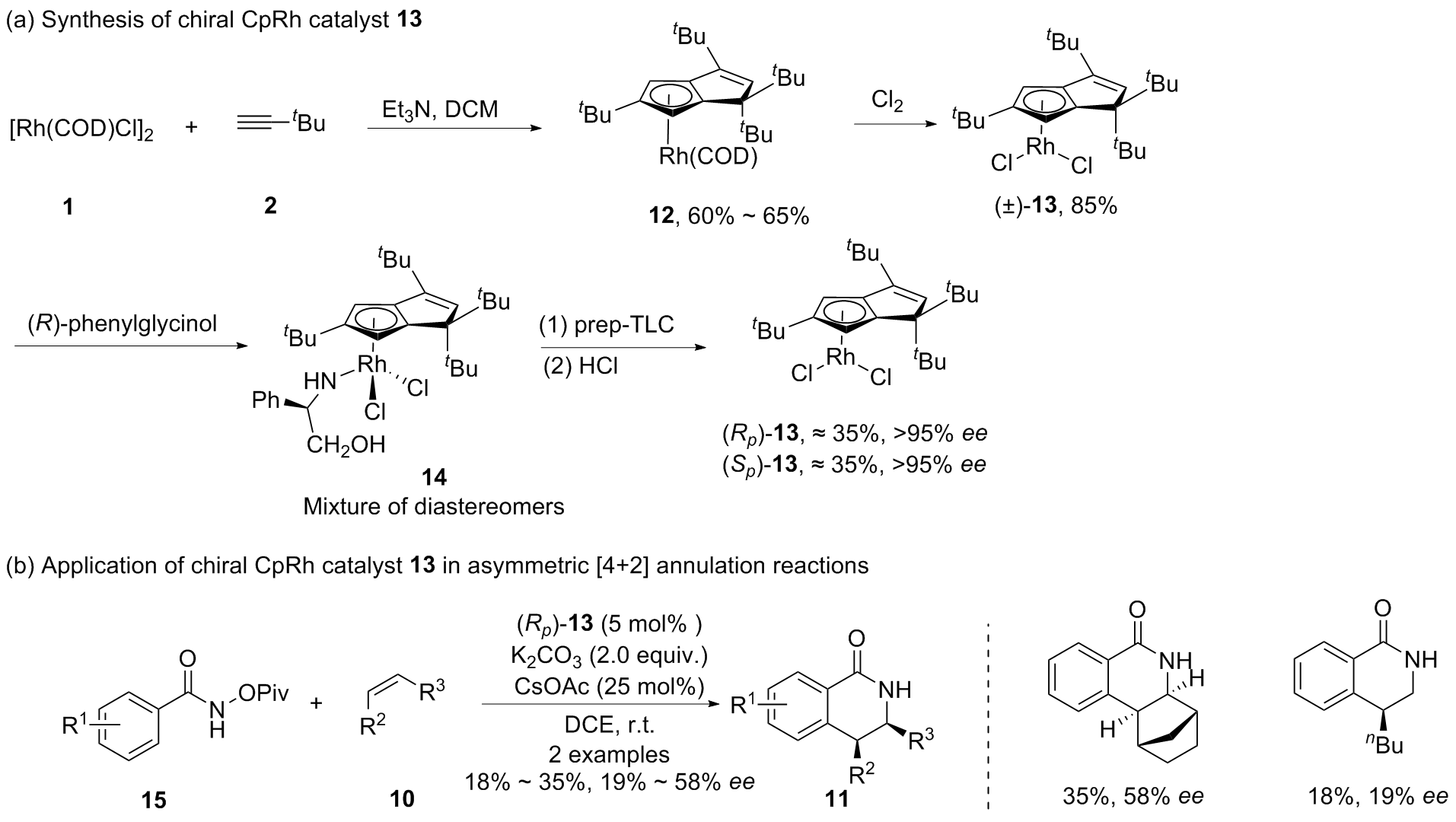
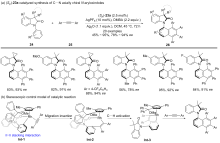
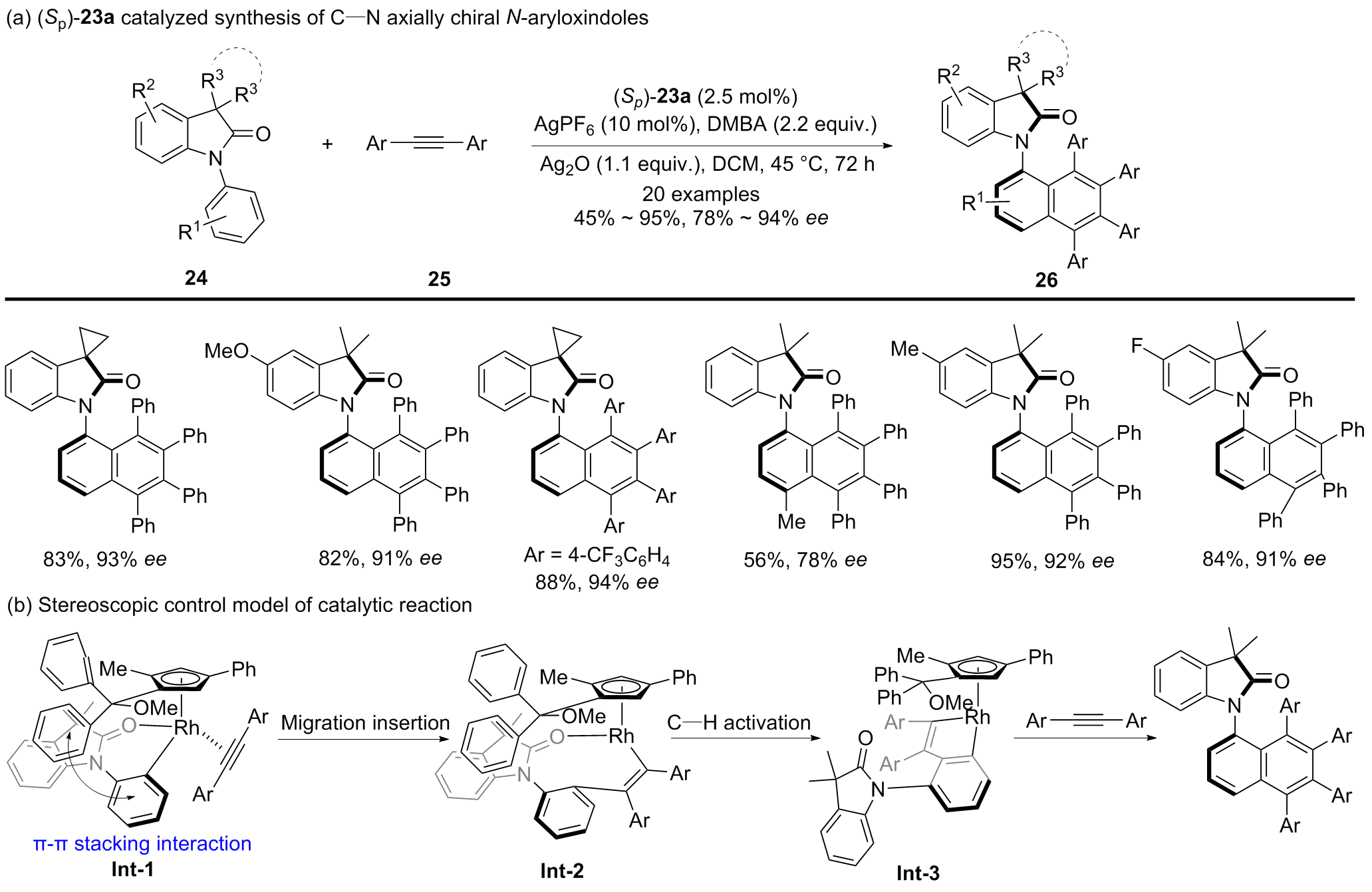
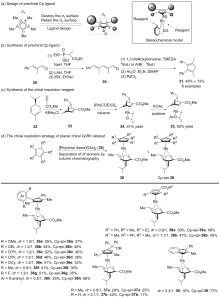

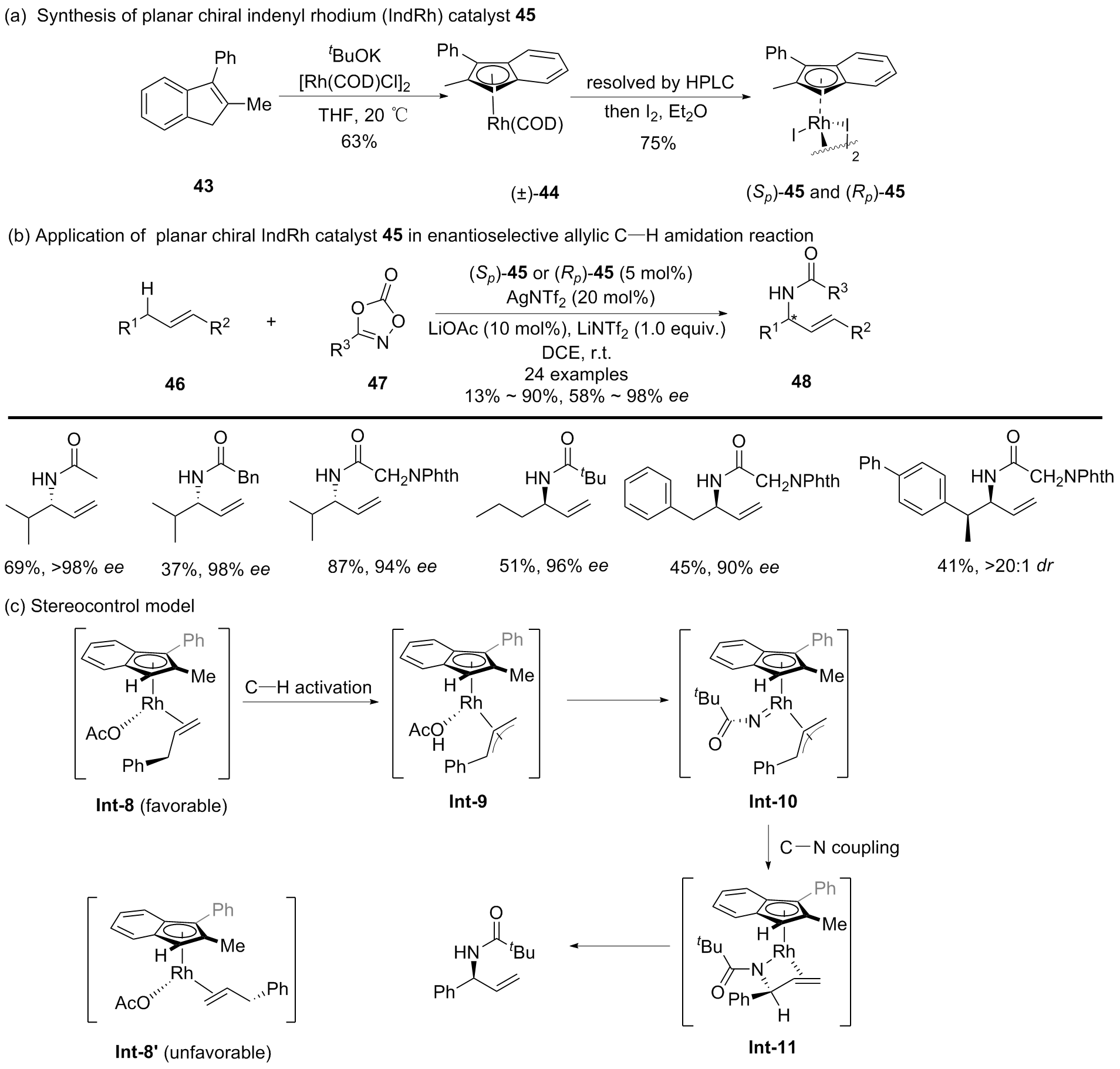
| [1] |
(a) Liu, C.-X.; Yin, S.-Y.; Zhao, F.; Yang, H.; Feng, Z.; Gu, Q.; You, S.-L. Chem. Rev. 2023, 123, 10079.
pmid: 32897713 |
|
(b) Liang, H.; Wang, J. Chem.-Eur. J. 2023, 29, e202202461.
pmid: 32897713 |
|
|
(c) Zheng, Y.; Zheng, C.; Gu, Q.; You, S.-L. Chem Catal. 2022, 2, 2965.
pmid: 32897713 |
|
|
(d) Zhang, Q.; Wu, L.-S.; Shi, B.-F. Chem 2022, 8, 384.
pmid: 32897713 |
|
|
(e) Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B.-F. Chem. Rev. 2021, 121, 14957.
pmid: 32897713 |
|
|
(f) Woźniak, Ł.; Tan, J.-F.; Nguyen, Q.-H.; Madron du Vigné, A.; Smal, V.; Cao, Y.-X.; Cramer, N. Chem. Rev. 2020, 120, 10516.
doi: 10.1021/acs.chemrev.0c00559 pmid: 32897713 |
|
|
(g) Woźniak, Ł.; Cramer, N. Trends Chem. 2019, 1, 471.
pmid: 32897713 |
|
|
(h) Loup, J.; Dhawa, U.; Pesciaioli, F.; Wencel-Delord, J.; Ackermann, L. Angew. Chem., Int. Ed. 2019, 58, 12803.
pmid: 32897713 |
|
|
(i) Liao, G.; Zhou, T.; Yao, Q.-J.; Shi, B.-F. Chem. Commun. 2019, 55, 8514.
pmid: 32897713 |
|
|
(j) Saint-Denis, T. G.; Zhu, R.-Y.; Chen, G.; Wu, Q.-F.; Yu, J.-Q. Science 2018, 359, eaao4798.
pmid: 32897713 |
|
|
(k) Newton, C. G.; Wang, S.-G.; Oliveira, C. C.; Cramer, N. Chem. Rev. 2017, 117, 8908.
pmid: 32897713 |
|
| [2] |
Hyster, T. K.; Knörr, L.; Ward, T. R.; Rovis, T. Science 2012, 338, 500.
doi: 10.1126/science.1226132 pmid: 23112327 |
| [3] |
Ye, B.; Cramer, N. Science 2012, 338, 504.
|
| [4] |
(a) Yan, X.; Wang, J. Synthesis 2023, 55, 1309.
|
|
(b) Satheeshkumar, A. K.; Usman, R.; Hapke, M. In Topics in Organometallic Chemistry, Springer Berlin Heidelberg, Berlin, Heidelberg, 2023, p. 1.
|
|
|
(c) Pan, Y.; Qin, X.; Yuan, C.; Lu, Y. Chin. J. Org. Chem. 2023, 43, 924 (in Chinese).
|
|
|
(潘彦年, 秦萧, 袁成凯, 鲁艺, 有机化学, 2023, 43, 924.)
doi: 10.6023/cjoc202211039 |
|
|
(d) Achar, T. K.; Al-Thabaiti, S. A.; Mokhtar, M.; Maiti, D. Chem Cat. 2023, 3, 100575.
|
|
|
(e) Wang, Q.; Liu, C.-X.; Gu, Q.; You, S.-L. Sci. Bull. 2021, 66, 210.
|
|
|
(f) Mas-Roselló, J.; Herraiz, A. G.; Audic, B.; Laverny, A.; Cramer, N. Angew. Chem., Int. Ed. 2021, 60, 13198.
|
|
|
(g) Yoshino, T.; Satake, S.; Matsunaga, S. Chem.-Eur. J. 2020, 26, 7346.
|
|
|
(h) Shaaban, S.; Davies, C.; Waldmann, H. Eur. J. Org. Chem. 2020, 2020, 6512.
|
|
| [5] |
Yan, X.; Jiang, J.; Wang, J. Angew. Chem., Int. Ed. 2022, 61, e202201522.
|
| [6] |
Wang, S. G.; Park, S. H.; Cramer, N. Angew. Chem., Int. Ed. 2018, 57, 5459.
|
| [7] |
(a) Wang, S.-G.; Cramer, N. ACS Catal. 2020, 10, 8231.
pmid: 29732080 |
|
(b) Cui, W. J.; Wu, Z. J.; Gu, Q.; You, S. L. J. Am. Chem. Soc. 2020, 142, 7379.
pmid: 29732080 |
|
|
(c) Sun, Y.; Cramer, N. Chem. Sci. 2018, 9, 2981.
doi: 10.1039/c7sc05411d pmid: 29732080 |
|
|
(d) Ye, B.; Cramer, N. J. Am. Chem. Soc. 2013, 135, 636.
pmid: 29732080 |
|
| [8] |
Duchemin, C.; Smits, G.; Cramer, N. Organometallics 2019, 38, 3939.
doi: 10.1021/acs.organomet.9b00365 |
| [9] |
Zheng, J.; Cui, W. J.; Zheng, C.; You, S. L. J. Am. Chem. Soc. 2016, 138, 5242.
doi: 10.1021/jacs.6b02302 pmid: 27070297 |
| [10] |
Pan, C.; Yin, S. Y.; Wang, S. B.; Gu, Q.; You, S. L. Angew. Chem., Int. Ed. 2021, 60, 15510.
|
| [11] |
Li, G.; Yan, X.; Jiang, J.; Liang, H.; Zhou, C.; Wang, J. Angew. Chem., Int. Ed. 2020, 59, 22436.
|
| [12] |
Liang, H.; Vasamsetty, L.; Li, T.; Jiang, J.; Pang, X.; Wang, J. Chem.-Eur. J. 2020, 26, 14546.
|
| [13] |
(a) Yang, H.; Zhang, R.; Zhang, S.-Z.; Gu, Q.; You, S.-L. ACS Catal. 2023, 13, 8838.
|
|
(b) Guo, W.; Pang, X.; Jiang, J.; Wang, J. Org. Lett. 2023, 25, 3823.
|
|
| [14] |
Guo, W.; Jiang, J.; Wang, J. Angew. Chem., Int. Ed. 2024, 63, e202400279.
|
| [15] |
Jia, Z. J.; Merten, C.; Gontla, R.; Daniliuc, C. G.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2017, 56, 2429.
|
| [16] |
Pototskiy, R. A.; Kolos, A. V.; Nelyubina, Y. V.; Perekalin, D. S. Eur. J. Org. Chem. 2020, 2020, 6019.
|
| [17] |
Kharitonov, V. B.; Podyacheva, E.; Chusov, D.; Nelyubina, Y. V.; Muratov, D. V.; Loginov, D. A. Org. Lett. 2023, 25, 8906.
doi: 10.1021/acs.orglett.3c03726 pmid: 38051945 |
| [18] |
Edlová, T.; Rybáček, J.; Cattey, H.; Vacek, J.; Bednárová, L.; Gendre, P. L.; Normand, A. T.; Stará, I. G.; Starý, I. Angew. Chem., nt. Ed. 2024, 63, e202414698.
|
| [19] |
Trifonova, E. A.; Ankudinov, N. M.; Mikhaylov, A. A.; Chusov, D. A.; Nelyubina, Y. V.; Perekalin, D. S. Angew. Chem., Int. Ed. 2018, 57, 7714.
|
| [20] |
Kolos, A. V.; Nelyubina, Y. V.; Sundararaju, B.; Perekalin, D. S. Organometallics 2021, 40, 3712.
|
| [21] |
Farr, C. M. B.; Kazerouni, A. M.; Park, B.; Poff, C. D.; Won, J.; Sharp, K. R.; Baik, M.-H.; Blakey, S. B. J. Am. Chem. Soc. 2020, 142, 13996.
|
| [22] |
Zhang, C.; Jiang, J.; Huang, X.; Wang, J. ACS Catal. 2023, 13, 10468.
|
| [23] |
Kolos, A. V.; Nelyubina, Y. V.; Podyacheva, E. S.; Perekalin, D. S. Dalton Trans. 2023, 52, 17005.
|
| [24] |
Gross, P.; Im, H.; Laws, D., III; Park, B.; Baik, M.-H.; Blakey, S. B. J. Am. Chem. Soc. 2024, 146, 1447.
|
| [1] | 乔秀秀, 李倩, 赵世娜, 魏瑞琪, 马桃, 何永辉, 赵晓静. 2-取代的3H-吲哚-3-酮类化合物参与的C2位手性吲哚啉-3-酮类化合物的不对称合成研究进展[J]. 有机化学, 2025, 45(4): 1166-1177. |
| [2] | 张晓锋, Aggeliki Roumana, 毛海康, 徐晶. 虎皮楠生物碱Daphniglaucin C的AB环系合成[J]. 有机化学, 2025, 45(3): 925-932. |
| [3] | 杨之同, 宋恒谦, 雷盼, 闫嘉航, 谢卫青. Communesin生物碱核心五环骨架的高效合成[J]. 有机化学, 2025, 45(3): 1003-1008. |
| [4] | 林恩泽, 李必杰. 基于碳氢键断裂的金属催化的内烯烃不对称氢芳基化进展[J]. 有机化学, 2025, 45(2): 546-558. |
| [5] | 王曼曼, 习文慧, 吴昊, 白大昌. 过渡金属催化碳氢键活化合成烷基氟烷基化合物的研究进展[J]. 有机化学, 2025, 45(2): 516-530. |
| [6] | 杨雪莹, 徐园双, 张新迎, 范学森. 基于2-芳基-3H-吲哚与环丙醇的串联反应合成C2-螺环吲哚啉衍生物[J]. 有机化学, 2025, 45(2): 694-706. |
| [7] | 张朝威, 徐兵斌, 刘文龙, 赵敬, 段伟良. 钯催化不对称碳氢键活化合成平面手性二茂铁磺酰胺化合物[J]. 有机化学, 2025, 45(2): 707-716. |
| [8] | 梅明顺, 张扬会. 钯催化惰性亚甲基C(sp3)—H键分子间官能团化反应[J]. 有机化学, 2025, 45(2): 620-640. |
| [9] | 张经明, 何智涛. 过渡金属催化远程二烯的不对称迁移烯丙位碳氢键官能团化[J]. 有机化学, 2025, 45(2): 592-601. |
| [10] | 杨帆, 范小梦, 姚雪婧, 米瑞杰, 于松杰, 李兴伟, 肖建. 铑(III)催化氧化锍叶立德和重氮化合物环化偶联反应[J]. 有机化学, 2025, 45(1): 331-342. |
| [11] | 金欢, 朱彦军, 李芳, 罗云飞. 新型含酯基官能团的茂基金属铑催化剂的合成及其在催化合成异喹啉反应中的应用[J]. 有机化学, 2024, 44(9): 2906-2914. |
| [12] | 温梦柯, 李宜越, 杜浩康, 陈章培, 杨西发. 铑催化3-芳基-2H-1,4-苯并噁嗪与重氮萘-2H-酮的[3+3]环化合成螺吡喃化合物[J]. 有机化学, 2024, 44(7): 2223-2232. |
| [13] | 于蕾, 盛康, 李亭, 唐从辉. 多相铁催化环状醚C(sp3)—H键活化的芳基烯烃氧烷基化[J]. 有机化学, 2024, 44(6): 1978-1986. |
| [14] | 王家晟, 王泽树, 何卫民, 叶龙武. 邻炔基苯胺氢胺化合成轴手性吲哚研究进展[J]. 有机化学, 2024, 44(6): 1786-1792. |
| [15] | 刘晨光. 含氮芳香性杂环化合物的不对称氢化反应研究进展[J]. 有机化学, 2024, 44(5): 1403-1422. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||