有机化学 ›› 2025, Vol. 45 ›› Issue (4): 1283-1296.DOI: 10.6023/cjoc202407024 上一篇 下一篇
研究论文
刘梦琴a, 陈贻庭a, 张榉元a, 周和烨a, 秦涛a,*( ), 刘彬a,b,*(
), 刘彬a,b,*( )
)
收稿日期:2024-09-04
修回日期:2024-10-10
发布日期:2024-11-08
基金资助:
Mengqin Liua, Yiting Chena, Juyuan Zhanga, Heye Zhoua, Tao Qina( ), Bin Liua,b(
), Bin Liua,b( )
)
Received:2024-09-04
Revised:2024-10-10
Published:2024-11-08
Contact:
* E-mail: Supported by:文章分享
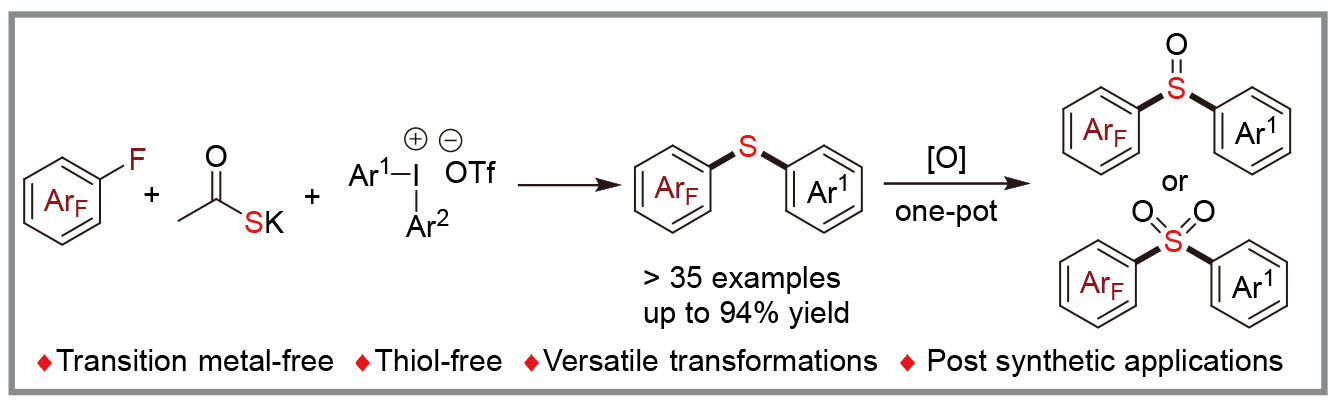
双芳基硫醚常存在于药物和天然产物中, 通常涉及金属交叉偶联反应、高温以及使用稳定性差、气味难闻的硫酚进行合成. 基于此, 研究了在无过渡金属催化条件下, 多氟芳烃、二芳基碘盐及硫代乙酸钾的三组分交叉偶联反应. 该反应通过商业化的硫代乙酸钾与两种缺电子芳烃经连续两次亲电取代反应, 合成了系列非对称的双芳基硫醚, 表现出了宽泛的底物适用范围和官能团兼容性. 此外, 通过一锅法氧化反应可以实现砜和亚砜类化合物的合成, 拓展了该策略的应用范围; 克级规模实验和衍生化实验均表现出优异的结果, 进一步证明了该反应具有潜在的应用价值. 通过对照、自由基抑制及中间体捕获实验阐明了其机制.
刘梦琴, 陈贻庭, 张榉元, 周和烨, 秦涛, 刘彬. 三组分交叉偶联反应高效合成双芳基硫醚[J]. 有机化学, 2025, 45(4): 1283-1296.
Mengqin Liu, Yiting Chen, Juyuan Zhang, Heye Zhou, Tao Qin, Bin Liu. Efficient Synthesis of Diaryl Sulfides via Three Component Cross Coupling[J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1283-1296.
| Entry | Molar ratio of 1a/2/3a | 2 | Temp./℃ | Solvent | Yieldb/% of 4a |
|---|---|---|---|---|---|
| 1c | 1/1.5/2 | 2a | 80 | DMSO | Trace |
| 2c | 1/1.5/2 | 2b | 80 | DMSO | 42 |
| 3 | 1/1.5/2 | 2c | 80 | DMSO | 69 |
| 4d | 1/1.5/2 | 2d | 80 | DMSO | 56 |
| 5 | 1/1.5/2 | 2e | 80 | DMSO | 55 |
| 6 | 1/1.5/2 | 2f | 80 | DMSO | 43 |
| 7 | 1/1.5/2 | 2c | 80 | DMF | 57 |
| 8 | 1/1.5/2 | 2c | 80 | Acetone | 43 |
| 9 | 1/1.5/2 | 2c | 80 | THF | Trace |
| 10 | 1/1.5/2 | 2c | 80 | CH3CN | Trace |
| 11 | 1/1.5/2 | 2c | 80 | Toluene | n.d. |
| 12 | 1/1.5/2 | 2c | 80 | DCE | n.d. |
| 13 | 1/1.5/2 | 2c | 60 | DMSO | 58 |
| 14 | 1/1.5/2 | 2c | 100 | DMSO | 73 |
| 15 | 1/2/2 | 2c | 100 | DMSO | 82 |
| 16 | 1/2/1.5 | 2c | 100 | DMSO | 61 |
| Entry | Molar ratio of 1a/2/3a | 2 | Temp./℃ | Solvent | Yieldb/% of 4a |
|---|---|---|---|---|---|
| 1c | 1/1.5/2 | 2a | 80 | DMSO | Trace |
| 2c | 1/1.5/2 | 2b | 80 | DMSO | 42 |
| 3 | 1/1.5/2 | 2c | 80 | DMSO | 69 |
| 4d | 1/1.5/2 | 2d | 80 | DMSO | 56 |
| 5 | 1/1.5/2 | 2e | 80 | DMSO | 55 |
| 6 | 1/1.5/2 | 2f | 80 | DMSO | 43 |
| 7 | 1/1.5/2 | 2c | 80 | DMF | 57 |
| 8 | 1/1.5/2 | 2c | 80 | Acetone | 43 |
| 9 | 1/1.5/2 | 2c | 80 | THF | Trace |
| 10 | 1/1.5/2 | 2c | 80 | CH3CN | Trace |
| 11 | 1/1.5/2 | 2c | 80 | Toluene | n.d. |
| 12 | 1/1.5/2 | 2c | 80 | DCE | n.d. |
| 13 | 1/1.5/2 | 2c | 60 | DMSO | 58 |
| 14 | 1/1.5/2 | 2c | 100 | DMSO | 73 |
| 15 | 1/2/2 | 2c | 100 | DMSO | 82 |
| 16 | 1/2/1.5 | 2c | 100 | DMSO | 61 |
| [1] |
(a) Liu, H.; Fujiwara, T.; Nishikawa, T.; Mishima, Y.; Nagai, H.; Shida, T.; Tachibana, K.; Kobayashi, H.; Mangindaan, R. E.; Namikoshi, M. Tetrahedron 2005, 61, 8611.
pmid: 28418240 |
|
(b) Nakazawa, T.; Xu, J.; Nishikawa, T.; Oda, T.; Fujita, A.; Ukai, K.; Mangindaan, R. E.; Rotinsulu, H.; Kobayashi, H.; Namikoshi, M. J. Nat. Prod. 2007, 70, 439.
pmid: 28418240 |
|
|
(c) Dunbar, K. L.; Scharf, D. H.; Litomska, A.; Hertweck, C. Chem. Rev. 2017, 117, 5521.
doi: 10.1021/acs.chemrev.6b00697 pmid: 28418240 |
|
|
(d) Iino, H.; Usui, T.; Hanna, J. Nat. Commun. 2015, 6, 1.
pmid: 28418240 |
|
|
(e) Roy, K. M. Ullmannʼs Encycl. Ind. Chem. 2000, 36, 625.
pmid: 28418240 |
|
| [2] |
Mao, Y.; Jiang, L.; Chen, T.; He, H.; Liu, G.; Wang, H. Synthesis 2015, 47, 1387.
|
| [3] |
Chekal, B. P.; Guinness, S. M.; Lillie, B. M.; McLaughlin, R. W.; Palmer, C. W.; Post, R. J.; Sieser, J. E.; Singer, R. A.; Sluggett, G. W.; Vaidyanathan, R. Org. Process Res. Dev. 2014, 18, 266.
|
| [4] |
Moreno, E.; Calvo, A.; Schwartz, J.; Navarro-Blasco, I.; González-Peñas, E.; Sanmartín, C.; Irache, J. M.; Espuelas, S. Pharmaceutics 2019, 11, 607.
|
| [5] |
Scott, K. A.; Njardarson, J. T. Top. Curr. Chem. 2018, 376, 1.
|
| [6] |
(a) Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291.
|
|
(b) Zhang, B.; Kang, Y.; Shi, R. Chin. J. Org. Chem. 2016, 36, 1814 (in Chinese).
|
|
|
(张变香, 亢永强, 史瑞雪, 有机化学, 2016, 36, 1814.)
doi: 10.6023/cjoc201602021 |
|
|
(c) Abedinifar, F.; Bahadorikhalili, S.; Larijani, B.; Mahdavi, M.; Verpoort, F. Appl. Organomet. Chem. 2022, 36, e6482.
|
|
|
(d) Qin, S. Chin. J. Org. Chem. 2023, 43, 3761 (in Chinese).
|
|
|
(秦思凝, 有机化学, 2023, 43, 3761.)
doi: 10.6023/cjoc202303042 |
|
|
(e) Parumala, S. K. R.; Surasani, S. R.; Peddinti, R. K. New J. Chem. 2014, 38, 5268.
|
|
|
(f) Zhou, Q.; Zhang, B.; Gu, H.; Zhong, A.; Du, T.; Zhou, Q.; Ye, Y.; Jin, Z.; Jiang, H.; Chen, R. Lett. Org. Chem. 2012, 9, 175.
|
|
|
(g) Zhang, Y.; Wang, Y.; Wang, L.; Han, J. Org. Biomol. Chem. 2024, 22, 486.
|
|
| [7] |
Jang, H. Org. Biomol. Chem. 2021, 19, 8656.
|
| [8] |
(a) Li, Y.; Liu, L.; Shan, D.; Liang, F.; Wang, S.; Yu, L.; Liu, J.-Q.; Wang, Q.; Shao, X.; Zhu, D. ACS Catal. 2023, 13, 13474.
|
|
(b) Liu, Y.; Xing, S.; Zhang, J.; Liu, W.; Xu, Y.; Zhang, Y.; Yang, K.; Yang, L.; Jiang, K.; Shao, X. Org. Chem. Front. 2022, 9, 1375.
|
|
|
(c) Zhang, W.; Huang, M.; Zou, Z.; Wu, Z.; Ni, S.; Kong, L.; Zheng, Y.; Wang, Y.; Pan, Y. Chem. Sci. 2021, 12, 2509.
|
|
|
(d) Graßl, S.; Hamze, C.; Koller, T. J.; Knochel, P. Chem.-Eur. J. 2019, 25, 3752.
|
|
|
(e) Dong, Z.-B.; Balkenhohl, M.; Tan, E.; Knochel, P. Org. Lett. 2018, 20, 7581.
|
|
|
(f) Wang, X.; Meng, J.; Zhao, D.; Tang, S.; Sun, K. Chin. Chem. Lett. 2023, 34, 107736.
|
|
| [9] |
(a) Wang, X.; Chen, J.-Q.; Yang, X.-X.; Hao, E.-J.; Dong, Z.-B. Eur. J. Org. Chem. 2022, e202200015.
pmid: 23327334 |
|
(b) Li, F.; Wang, D.; Chen, H.; He, Z.; Zhou, L.; Zeng, Q. Chem. Commun. 2020, 56, 13029.
pmid: 23327334 |
|
|
(c) Zou, L.-H.; Zhao, C.; Li, P.-G.; Wang, Y.; Li, J. J. Org. Chem. 2017, 82, 12892.
pmid: 23327334 |
|
|
(d) Prasad, C. D.; Balkrishna, S. J.; Kumar, A.; Bhakuni, B. S.; Shrimali, K.; Biswas, S.; Kumar, S. J. Org. Chem. 2013, 78, 1434.
doi: 10.1021/jo302480j pmid: 23327334 |
|
|
(e) Fernández-Salas, J. A.; Pulis, A. P.; Procter, D. J. Chem. Commun. 2016, 52, 12364.
pmid: 23327334 |
|
|
(f) Xia, D.; Luo, J.; He, L.; Cai, Z.; Du, G. Chin. J. Org. Chem. 2024, 44, 622 (in Chinese).
pmid: 23327334 |
|
|
(夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬, 有机化学, 2024, 44, 622.)
doi: 10.6023/cjoc202306014 pmid: 23327334 |
|
| [10] |
(a) Bacchi, S.; Benaglia, M.; Cozzi, F.; Demartin, F.; Filippini, G.; Gavezzotti, A. Chem.-Eur. J. 2006, 12, 3538.
pmid: 26861673 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
pmid: 26861673 |
|
|
(c) Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496.
pmid: 26861673 |
|
|
(d) Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052.
pmid: 26861673 |
|
|
(e) Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
doi: 10.1021/acs.chemrev.5b00547 pmid: 26861673 |
|
| [11] |
(a) Liu, C.; Zhang, B. Chem. Rec. 2016, 16, 667.
pmid: 28793179 |
|
(b) Li, X.; Fu, B.; Zhang, Q.; Yuan, X.; Zhang, Q.; Xiong, T.; Zhang, Q. Angew. Chem., Int. Ed. 2020, 59, 23056.
pmid: 28793179 |
|
|
(c) Zhao, Y.; Fu, B.; Wang, S.; Li, Y.; Yuan, X.; Yin, J.; Xiong, T. Zhang, Q. Org. Lett. 2023, 25, 2492.
doi: 10.1021/acs.orglett.3c00671 pmid: 28793179 |
|
|
(d) Linde, E.; Bulfield, D.; Kervefors, G.; Purkait, N.; Olofsson, B. Chem 2022, 8, 850.
pmid: 28793179 |
|
|
(e) Stuart, D. R. Chem.-Eur. J. 2017, 23, 15852.
doi: 10.1002/chem.201702732 pmid: 28793179 |
|
|
(f) Fañanás-Mastral, M. Synthesis 2017, 49, 1905.
pmid: 28793179 |
|
| [12] |
(a) Yang, L.; Qin, T.; Liu, B. Synlett 2023, 34, 2336.
|
|
(b) Liao, W.; Du, H.; Chen, M.; Xiong, Y.; Zhou, H.; Qin, T.; Liu, B. Org. Biomol. Chem. 2024, 22, 708.
|
|
| [13] |
(a) Fortugno, C.; Varchi, G.; Guerrini, A. Biomed. Anal. 2014, 95, 151.
|
|
(b) Gambini, L.; Udompholkul, P.; Salem, A. F.; Baggio, C.; Pellecchia, M. ChemMedChem 2020, 15, 2176.
|
|
|
(c) Tang, X.; Zhang, N.; He, G.; Li, C.-H.; Huang, W.; Wang, X.-Y.; Zhan, G.; Han, B. Org. Lett. 2020, 22, 7909.
|
|
| [14] |
(a) Mondal, S.; Di Tommaso, E. M.; Olofsson, B. Angew. Chem., Int. Ed. 2023, 62, No. e202216296.
|
|
(b) Wu, S.; Wong, T. H.-F.; Righi, P.; Melchiorre, P. J. Am. Chem. Soc. 2024, 146, 2907.
|
|
| [15] |
Wang, D.; Yu, X.; Zhao, K.; Li, L.; Ding, Y. Tetrahedron Lett. 2014, 55, 5739.
|
| [1] | 谢沈彤, 李文静, 刘钰, 陆熹, 师仁义. 镍催化多氟芳烃与烷基卤化物的还原烷基化反应[J]. 有机化学, 2025, 45(6): 2121-2127. |
| [2] | 黄真茹, 金国顺, 陈天煜, 冯斌, 史鑫康, 陈敏方, 华路生, 徐清. 氢氧化铯催化温和有氧环化反应高效构建喹喔啉杂环衍生物[J]. 有机化学, 2024, 44(9): 2933-2942. |
| [3] | 田永盛, 魏斓枫, 黄嘉为, 韦玉, 徐亮, 刘帅. 四丁基三溴化铵促进的有机硼酸在无过渡金属条件下的脱硼硒化、溴化和羟基化反应[J]. 有机化学, 2024, 44(6): 1987-1997. |
| [4] | 鞠国栋, 周冠宇, 赵应声. 三异丙基硅烷(TIPS)保护苯酚的无过渡金属催化区域选择性硫氰化反应[J]. 有机化学, 2024, 44(4): 1327-1336. |
| [5] | 万云辉, 杨福美, 陈明瀚, 孙德立, 叶丹锋. 无过渡金属催化的N-苄基-N-叔丁氧羰基酰胺与不饱和醇的酯化反应[J]. 有机化学, 2024, 44(4): 1293-1300. |
| [6] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [7] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [8] | 徐忠荣, 万结平, 刘云云. 基于热、光以及电化学过程的无过渡金属碳-氢键硫氰化和硒氰化反应[J]. 有机化学, 2023, 43(7): 2425-2446. |
| [9] | 秦娇, 陈杰, 苏艳. 无过渡金属催化的α-溴代茚酮自由基裂解反应合成(2-氰基苯基)乙酸-2,2,6,6-四甲基哌啶酯[J]. 有机化学, 2023, 43(6): 2171-2177. |
| [10] | 王睿, 高朗, 周岑, 张霄. 苯基吩噻嗪多孔有机聚合物催化的非活化末端烯烃的卤代全氟烷基化反应[J]. 有机化学, 2023, 43(3): 1136-1145. |
| [11] | 孙婧, 张萌萌, 锅小龙, 王琪, 王陆瑶. 无过渡金属条件下二芳基硒化合物的合成[J]. 有机化学, 2023, 43(12): 4251-4260. |
| [12] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| [13] | 陈天煜, 韩峰, 李双艳, 刘建平, 陈建辉, 徐清. 无过渡金属参与杂环甲基化合物与醇的选择性有氧碳-烷基化反应[J]. 有机化学, 2022, 42(9): 2914-2924. |
| [14] | 许耀辉, 吴镇, 吴新鑫, 朱晨. 无过渡金属参与的醚、醛和酰胺C—H键自由基炔基化和烯丙基化反应[J]. 有机化学, 2022, 42(12): 4340-4349. |
| [15] | 叶丹锋, 陈浩, 刘志园, 雷川虎. 无过渡金属及无碱参与的N-苄基-N-叔丁基羰基酰胺的转酰胺化[J]. 有机化学, 2021, 41(4): 1658-1669. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||