有机化学 ›› 2025, Vol. 45 ›› Issue (8): 2968-2982.DOI: 10.6023/cjoc202411004J 上一篇 下一篇
研究论文
收稿日期:2025-01-23
修回日期:2025-02-21
发布日期:2025-03-13
基金资助:
Xiu Xue, Huier Yang, Zeshen Huang, Ming Lang*( ), Wenfeng Liu*(
), Wenfeng Liu*( )
)
Received:2025-01-23
Revised:2025-02-21
Published:2025-03-13
Contact:
*E-mail:langm2019@163.com;wyuchemlwf@126.com
Supported by:文章分享
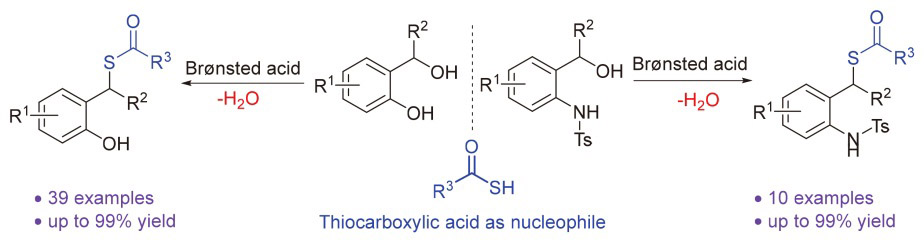
报道了一种布朗斯特酸催化下硫代羧酸与原位生成的邻亚甲基醌和氮杂邻亚甲基醌之间的共轭加成反应. 该反应具有底物适用范围广、反应条件温和、操作简单和产率高的优点, 为在温和条件下高效构建具有结构多样性的硫酯类化合物提供了新的方法.
薛秀, 杨惠儿, 黄泽深, 郎明, 刘文锋. 布朗斯特酸催化下硫代羧酸与(氮杂)邻亚甲基醌的共轭加成反应[J]. 有机化学, 2025, 45(8): 2968-2982.
Xiu Xue, Huier Yang, Zeshen Huang, Ming Lang, Wenfeng Liu. Brønsted Acid Catalyzed Conjugate Addition of Thiocarboxylic Acids to in situ-Generated (aza-)ortho-Quinone Methides[J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2968-2982.
| Entry | Cat. | Solvent | Yieldb/% | Entry | Cat. | Solvent | Yieldb/% |
|---|---|---|---|---|---|---|---|
| 1 | A | CH2Cl2 | >99 | 9 | A | CH3CO2Et | 47 |
| 2 | B | CH2Cl2 | 48 | 10 | A | CH3COCH3 | 39 |
| 3 | C | CH2Cl2 | Trace | 11 | A | CH3C6H5 | 88 |
| 4 | D | CH2Cl2 | 99 | 12 | A | ClC6H5 | 94 |
| 5 | E | CH2Cl2 | 95 | 13 | A | | Trace |
| 6 | F | CH2Cl2 | 73 | 14 | A | | Trace |
| 7 | G | CH2Cl2 | Trace | 15 | — | CH2Cl2 | 0 |
| 8 | A | CH3CN | 54 |
| Entry | Cat. | Solvent | Yieldb/% | Entry | Cat. | Solvent | Yieldb/% |
|---|---|---|---|---|---|---|---|
| 1 | A | CH2Cl2 | >99 | 9 | A | CH3CO2Et | 47 |
| 2 | B | CH2Cl2 | 48 | 10 | A | CH3COCH3 | 39 |
| 3 | C | CH2Cl2 | Trace | 11 | A | CH3C6H5 | 88 |
| 4 | D | CH2Cl2 | 99 | 12 | A | ClC6H5 | 94 |
| 5 | E | CH2Cl2 | 95 | 13 | A | | Trace |
| 6 | F | CH2Cl2 | 73 | 14 | A | | Trace |
| 7 | G | CH2Cl2 | Trace | 15 | — | CH2Cl2 | 0 |
| 8 | A | CH3CN | 54 |
| [1] |
(a)
pmid: 26813743 |
|
(b)
pmid: 26813743 |
|
|
(c)
doi: 10.1111/cns.12495 pmid: 26813743 |
|
|
(d)
pmid: 26813743 |
|
|
(e)
pmid: 26813743 |
|
| [2] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(梁陆祺, 奚娟, 姜若楠, 杨艺, 孙丰钢, 张立志, 李新进, 刘会, 有机化学, 2023, 43, 1566.)
doi: 10.6023/cjoc202208035 |
|
|
(h)
|
|
|
韩明亮, 徐丽华, 化学学报 2023, 81, 381).
doi: 10.6023/A23010013 |
|
|
(i)
|
|
|
(王晓晨, 季泽尧, 刘健, 王炳福, 金辉, 张立新, 化学学报 2023, 81, 64.)
doi: 10.6023/A22100422 |
|
| [3] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [4] |
(a)
doi: 10.1002/chem.201101073 pmid: 21735501 |
|
(b)
pmid: 21735501 |
|
| [5] |
(a)
pmid: 31247799 |
|
(b)
doi: 10.1021/acs.orglett.9b01774 pmid: 31247799 |
|
|
(c)
pmid: 31247799 |
|
|
(d)
pmid: 31247799 |
|
|
(e)
pmid: 31247799 |
|
|
(f)
pmid: 31247799 |
|
|
(g)
pmid: 31247799 |
|
|
(h)
pmid: 31247799 |
|
|
(i)
pmid: 31247799 |
|
| [6] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [7] |
|
| [8] |
(a)
|
|
(b)
|
|
| [9] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [10] |
|
| [11] |
|
|
(王海清, 杨爽, 张宇辰, 石枫, 有机化学, 2023, 43, 974.)
doi: 10.6023/cjoc202211022 |
|
| [12] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [13] |
|
| [14] |
|
| [15] |
doi: 10.1016/j.tetlet.2019.06.061 |
| [16] |
(a)
doi: 10.1021/acs.orglett.1c03402 pmid: 30346172 |
|
(b)
doi: 10.1021/acs.joc.8b02470 pmid: 30346172 |
|
| [17] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [18] |
(a)
doi: 10.1021/jo402132p pmid: 24255969 |
|
(b)
pmid: 24255969 |
| [1] | 焦俊强, 杨高升. α-[二(烷氧羰基)甲基]查尔酮的制备及其在多取代环丙烷衍生物合成中的应用[J]. 有机化学, 2025, 45(7): 2586-2599. |
| [2] | 杨之同, 宋恒谦, 雷盼, 闫嘉航, 谢卫青. Communesin生物碱核心五环骨架的高效合成[J]. 有机化学, 2025, 45(3): 1003-1008. |
| [3] | 陈文龙, 李慧敏, 杨鹏飞, 郑东程, 杨高升. 2-芳甲酰基甲亚基丙二酸酯与Corey叶立德的反应[J]. 有机化学, 2023, 43(4): 1472-1482. |
| [4] | 王兴, 宋倩倩, 陈续玲, 李鹏飞, 齐昀坤, 李文军. 有机催化远程立体控制6-亚甲基-6H-吲哚与异噁唑-5(4H)-酮的氮杂1,8-共轭加成反应[J]. 有机化学, 2022, 42(6): 1722-1734. |
| [5] | 李华, 庞靖祥, 刘华铮, 赵长印, 李松, 王恒山, 刘希功. Sc(OTf)3催化的δ-三氟甲基-δ-芳基-双取代对亚甲基苯醌和硫醇的反应: 高效合成二芳基甲烷硫醚化合物[J]. 有机化学, 2021, 41(8): 3134-3143. |
| [6] | 王东琳, 阚玲珑, 马玉道, 刘磊. 叔丁醇钠催化的δ-腈基-δ-芳基-双取代的对亚甲基苯醌和二芳基氧磷的磷氢化反应研究[J]. 有机化学, 2021, 41(8): 3192-3203. |
| [7] | 张同飞, 陈逸波, 高振博. 三氟甲磺酸铜(II)催化的硫醇对烯酮的共轭加成反应[J]. 有机化学, 2021, 41(6): 2424-2434. |
| [8] | 余述燕, 高丽宏, 兰宏兵, 钱恒玉, 尹志刚, 商永嘉. 橙酮衍生氮杂二烯的化学反应进展[J]. 有机化学, 2020, 40(9): 2714-2724. |
| [9] | 王琳, 王楠, 齐越, 孙书涛, 刘希功, 李伟, 刘磊. 基于δ-腈基取代对亚甲基苯醌1,6-氮杂共轭加成的大位阻α-氰胺合成研究[J]. 有机化学, 2020, 40(11): 3934-3943. |
| [10] | 刘腾, 刘建军, 贺池先, 成飞翔. 多共轭硝基二烯炔/硝基烯炔的合成以及应用研究进展[J]. 有机化学, 2017, 37(10): 2609-2618. |
| [11] | 邢爱萍, 田密, 王来来. C3对称的新型单齿亚磷酸酯配体在不对称氢甲酰化和1,4-共轭加成中的应用研究[J]. 有机化学, 2016, 36(12): 2912-2919. |
| [12] | 李明, 宁加彬, 于乐, 文丽荣. 铜促进硫代色酮类化合物的合成研究[J]. 有机化学, 2016, 36(11): 2715-2722. |
| [13] | 唐凤翔, 叶久勇. 铜催化格氏试剂不对称共轭加成研究进展[J]. 有机化学, 2015, 35(7): 1414-1427. |
| [14] | 吴春蕊, 杨玉坡, 史峰. 苯炔对双键插入反应的研究进展[J]. 有机化学, 2015, 35(4): 770-780. |
| [15] | 林敬, 程宇, 康泰然, 何龙, 刘全忠. 不饱和酮亚胺叶立德的分子内共轭加成[J]. 有机化学, 2014, 34(4): 735-740. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||