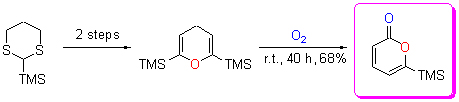
| [1] Tang, H.-Y.; Zeng, Y.; Li, Y.-Y.; Chen, J.-P.; Li, Y. Acta Chim. Sinica 2011, 69, 2241 (in Chinese). (唐海云, 曾毅, 李迎迎, 陈金平, 李嫕, 化学学报, 2011, 69, 2241.) [2] Deng, Y.; Balunas, M. J.; Kim, J. A.; Lantvit, D. D.; Chin, Y. W.; Chin, H.; Sugiarso, S.; Kardono, L. B. S.; Fong, H. H. S.; Penzzuto, J. M.; Swanson, S. M.; Blanco, E. J. C.; Kinghorm, A. D. J. Nat. Prod. 2009, 72, 1165. [3] Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. M. J. Nat. Prod. 2011, 74, 2014. [4] Breda, S.; Reva, I.; Lapinski, L.; Cristiano, M. L. S.; Fausto, R. J. Phys. Chem. A 2006, 110, 6415. [5] Sunazuka, T.; Omura, S. Chem. Rev. 2005, 105, 4559. [6] Romero, D. L.; Manninen, P. R.; Han, F.; Romero, A. G. J. Org. Chem. 1999, 64, 4980. [7] Tosaki, S. Y.; Nemoto, T.; Ohshima, T.; Shibasaki, M. Org. Lett. 2003, 5, 495. [8] Peng, D.-Q.; Liu, Y.; Lv, Z.-F.; Xu, J.-H. Chin. J. Org. Chem. 2009, 29, 716 (in Chinese). (彭大权, 刘蕴, 吕志锋, 徐建华, 有机化学, 2009, 29, 716.) [9] Cho, C. G.; Kim, Y. W.; Lim, Y. K.; Park, J. S.; Lee, H.; Koo, S. J. Org. Chem. 2002, 67, 290. [10] Lanari, D.; Ballini, R.; Palmieri, A.; Pizzo, F.; Vaccaro, L. Eur. J. Org. Chem. 2011, 2874. [11] Ohkata, K.; Lee, Y. G.; Utsumi, Y.; Ishimaru, K.; Akiba, K. Y. J. Org. Chem. 1991, 56, 5052. [12] Perkin, W. H. J. Chem. Soc. 1868, 21, 181-186. [13] Holden, M. S.; Crouch, R. D. J. Chem. Educ. 1998, 75, 1631. [14] Larock, R. C.; Han, X. J.; Doty, M. J. Tetrahedron Lett. 1998, 39, 5713. [15] Kotora, M.; Ishikawa, M.; Tsai, F. Y.; Takahashi, T. Tetrahedron 1999, 55, 4969 [16] Larock, R. C.; Doty, M. J.; Han X. J. J Org. Chem. 1999, 64, 8770. [17] Chatadaj, W.; Corbet, M.; Furstnerm, A. Angew. Chem., Int. Ed. 2012, 51, 6929. [18] Tsuda, T.; Morikawa, S.; Hasegawa, N.; Saegusa, T. J Org. Chem., 1990, 55, 2978. [19] Huang, Q.; Campo, M. A.; Yao, T.; Tian, Q.; Larock, R. C. J. Org. Chem. 2004, 69, 8251. [20] Yao, T.; Campo, M. A.; Larock, R. C. Org. Lett. 2004, 6, 2677. [21] Saleur, D.; Bouillon, J. P.; Portella, C.; Hoffmann, N. Tetrahedron Lett. 2000, 41, 5199. [22] Bouillon, J. P.; Portella, C. Eur. J. Org. Chem. 1999, 1571. [23] Chuang, T. H.; Fang, J. M.; Jiaang, W. T.; Tsai, Y. M. J. Org. Chem. 1996, 61, 1794. |