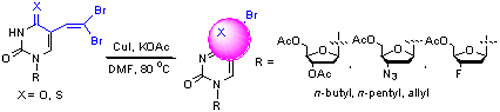
| [1] (a) De Clercq, E. Rev. Med. Virol. 2009, 19, 287. (b) De Clercq, E. J. Med. Chem. 2010, 53, 1438. [2] Zhang, X.-Y.; Wang, Y.-Y.; Feng, D.; Fan, X.-S. Chin. J. Org. Chem. 2010, 30, 797 (in Chinese). (张新迎, 王洋洋, 冯东, 范学森, 有机化学, 2010, 30, 797.) [3] Topalis, D.; Pradère, U.; Roy, V.; Caillat, C.; Azzouzi, A.; Broggi, J.; Snoeck, R.; Andrei, G.; Lin, J.; Eriksson, S.; Alexandre, J. A. C.; El-Amri, C.; Deville-Bonne, D.; Meyer, P.; Balzarini, J.; Agrofoglio, L. A. J. Med. Chem. 2011, 54, 222. [4] Srivastav, N. C.; Mak, M.; Agrawal, B.; Tyrrell, D. L. J.; Kumar, R. Bioorg. Med. Chem. Lett. 2010, 20, 6790. [5] McGuigan, C.; Yarnold, C. J.; Jones, C.; Velázquez, S.; Barucki, H.; Brancale, A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. J. Med. Chem. 1999, 42, 4479. [6] Balzarini, J.; McGuigan, C. J. Antimicrob. Chemother. 2002, 50, 5. [7] Janeba, Z.; Balzarini, J.; Andrei, G.; Snoeck, R.; De Clercq, E.; Robins, M. J. J. Med. Chem. 2005, 48, 4690. [8] Januszczyk, P.; Fogt, J.; Boryski, J.; Izawa, K.; Onishi, T.; Neyts, J.; De Clercq, E. Nucleosides Nucleotides Nucleic Acids 2009, 28, 713. [9] Kifli, N.; De Clercq, E.; Balzarini, J.; Simons, C. Bioorg. Med. Chem. 2004, 12, 4245. [10] Robins, M. J.; Barr, P. J. J. Org. Chem. 1983, 48, 1854. [11] Jin, Y.-Y.; Xiao, Q.; Ju, Y. Chin. J. Org. Chem. 2009, 29, 44 (in Chinese).(靳玄烨, 肖强, 巨勇, 有机化学, 2009, 29, 44.) [12] McGuigan, C.; Barucki, H.; Blewett, S.; Carangio, A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. J. Med. Chem. 2000, 43, 4993. [13] De Clercq, E. Med. Res. Rev. 2009, 29, 571. [14] Migliore, M. Antiviral Chem. Chemother. 2010, 20, 107. [15] Robins, M. J.; Miranda, K.; Rajwanshi, V. K.; Peterson, M. A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. J. Med. Chem. 2006, 49, 391. [16] Newman, S. G.; Aureggi, V.; Bryan, C. S.; Lautens, M. Chem. Commun. 2009, 5236. [17] Chen, W.; Zhang, Y.-C.; Zhang, L.; Wang, M.; Wang, L. Chem. Commun. 2011, 10476. [18] Fan, X.-S.; Wang, Y.-Y.; Qu, Y.-Y.; Xu, H.-Y.; He, Y.; Zhang, X.-Y.; Wang, J.-J. J. Org. Chem. 2011, 76, 982. [19] Desai, N. B.; McKelvie, N.; Ramirez, F. J. Am. Chem. Soc. 1962, 84, 1745.Corey, E. J.; Fuchs, P. L. Tetrahedron Lett. 1972, 13, 3769. |