| [1] Yet, L. Chem. Rev. 2003, 103, 4283.
[2] (a) Moore, R. E.; Bartolini, G. J. Am. Chem. Soc. 1981, 103, 2491. (b) Kagamizono, T.; Sakai, N.; Arai, K.; Kobinata, K.; Osada, H. Tetrahedron Lett. 1997, 38, 1223.
(c) Toske, S. G.; Jensen, P. R.; Kauffman, C. A.; Fencial, W. Tetrahedron 1998, 54, 13459.
(d) Davyt, D.; Entz, W.; Fernandez, R.; Mariezcurrena, R.; Mombru, A. W.; Saldana, J.; Dominguez, L.; Coll, J.; Manta, E. J. Nat. Prod. 1998, 61, 1560.
[3] Salama, N. N.; Eddington, N. D.; Payne, T. L.; Wilson, K. R.; Scott, K. R. Curr. Med. Chem. 2004, 11, 2093.
[4] (a) Xiao, D.; Zhang, Z.; Zhang, X. Org. Lett. 1999, 1, 1679.
(b) Burk, M. J. Acc. Chem. Res. 2000, 33, 363.
(c) Sibi, M. P.; Asano, Y. J. Am. Chem. Soc. 2001, 123, 9708.
(d) Blaser, H.-U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Adv. Synth. Catal. 2003, 345, 103.
[5] (a) Huang, K.; Zhang, X.; Geng, H.; Li, S.-K.; Zhang, X. ACS Catal. 2012, 2, 1343.
(b) Geng, H.; Huang, K.; Sun, T.; Li, W.; Zhang, X.; Zhou, L.; Wu, W.; Zhang, X. J. Org. Chem. 2011, 76, 332.
[6] (a) Hommes, P.; Berlin, S.; Reissig, H.-U. Synthesis 2013, 3288.
(b) Shabana, R.; Rasmussen, J. B.; Lawesson, S.-O. Tetrahedron 1981, 37, 1819.
[7] (a) Gao, D.; Back, T. G. Chem. Eur. J. 2012, 18, 14828.
(b) Li, X.; Huang, L.; Chen, H.; Wu, W.; Huang, H.; Jiang, H. Chem. Sci. 2012, 3, 3463.
[8] Gayon, E.; Szymczyk, M.; Gérard, H.; Vrancken, E.; Campagne, J.-M. J. Org. Chem. 2012, 77, 9205.
[9] (a) Panda, N.; Jena, A. K.; Raghavender, M. ACS Catal. 2012, 2, 539.
(b) Lee, J. M.; Ahn, D.-S.; Jung, D. Y.; Lee, J.; Do, Y.; Kim, S. K.; Chang, S. J. Am. Chem. Soc. 2006, 128, 12954.
(c) Gogoi, J.; Gogoi, P.; Boruah, R. C. Eur. J. Org. Chem. 2014, 3483.
(d) Panda, N.; Mothkuri, R. J. Org. Chem. 2012, 77, 9407.
(e) Ding, R.; Zhang, Q.-C.; Xu, Y.-H.; Loh, T.-P. Chem. Commun. 2014, 50, 11661.
[10] (a) Liu, D.; Lei, A. Chem. Asian J. 2015, 10, 806.
(b) Wu, X.; Gong, J.; Qi, X. Org. Biomol. Chem. 2014, 12, 5807. (c) Finkbeiner, P.; Nachtsheim, B. J. Synthesis 2013, 45, 979.
[11] (a) Ma, L.; Wang, X.; Yu, W.; Han, B. Chem. Commun. 2011, 47, 11333.
(b) Chen, J.; Shao, Y.; Zheng, H.; Cheng, J.; Wan, X. J. Org. Chem. 2015, 80, 10734.
(c) Xie, J.; Jiang, H.; Cheng, Y.; Zhu, C. Chem. Commun. 2012, 48, 979.
[12] Gao, W.-C.; Hu, F.; Huo, Y.-M.; Chang, H.-H.; Li, X.; Wei, W.-L. Org. Lett. 2015, 17, 3914.
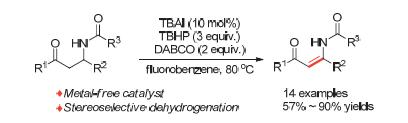
[13] The Z-isomer of products are confirmed by two evidences: (1) in H NMR spectrum, due to the intramolecular hydrogen bond formed between the amide group and the carbonyl group, the chemical shift of amide protons moves to the downfield relative to free N—H of amide, and in our reported products, the chemical shifts of N—H are all at about δ 10, different from normal chemical shifts of amide bond (δ 5~7); (2) According to Ref. [9e], the known structure of Z-isomer in 1a is consistent with our NMR data.
[14] Nicolaou, K. C.; Mathison, C. J. N. Angew. Chem., Int. Ed. 2005, 44, 5992.
[15] http://www.chem.wisc.edu/areas/reich/pkatable/index.htm
[16] Baidya, M.; Mayr, H. Chem. Commun. 2008, 1792.
[17] Sugiura, M.; Kumahara, M.; Nakajima, M. Chem. Commun. 2009, 3585. |