有机化学 ›› 2019, Vol. 39 ›› Issue (11): 3169-3175.DOI: 10.6023/cjoc201904028 上一篇 下一篇
研究论文
收稿日期:2019-04-10
发布日期:2019-07-09
通讯作者:
阿布拉江·克依木
E-mail:ablajan209@hotmail.com
基金资助:
Liang Jie, Ma Huifang, Ablajan Keyume*( )
)
Received:2019-04-10
Published:2019-07-09
Contact:
Ablajan Keyume
E-mail:ablajan209@hotmail.com
Supported by:文章分享

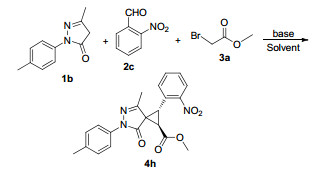
发展了一种4-二甲氨基吡啶(DMAP)促进的高立体选择性合成多取代螺环丙烷吡唑啉酮的方法.该反应以吡唑啉酮、芳醛和溴乙酸酯为原料,DMAP作为碱,经三组分一锅反应,合成一系列收率高且非对映选择性好的目标化合物.该反应具有操作简单、产率高以及非对映选择性好等优点.该合成方法对于螺环丙烷的研究具有重要的价值.
梁杰, 马会芳, 阿布拉江·克依木. 4-二甲氨基吡啶促进的一锅法合成高选择性螺环丙烷吡唑啉酮衍生物[J]. 有机化学, 2019, 39(11): 3169-3175.
Liang Jie, Ma Huifang, Ablajan Keyume. High-Selective One-Pot Synthesis of Spirocyclopropane Pyrazolones Promoted by 4-Dimethylaminopyridine[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3169-3175.
 | |||||
| Entry | Base | Solvent | Temp./℃ | Yieldb/% | transc/cis |
| 1 | No base | CH3CN | 81 | NR | NR |
| 2 | NaOH | CH3CN | 81 | 41 | 45:55 |
| 3 | Cs2CO3 | CH3CN | 81 | 48 | 48:52 |
| 4 | Pyridine | CH3CN | 81 | 82c | 72:28 |
| 5 | TEBA | CH3CN | 81 | 56 | 80:20 |
| 6 | CTAB | CH3CN | 81 | 60 | 85:15 |
| 7 | DABCO | CH3CN | 81 | 70 | 72:28 |
| 8 | DMAP | CH3CN | 81 | 86 | 94:6 |
| 9 | DMAP | CH2Cl2 | 81 | 65 | 40:60 |
| 10 | DMAP | H2O | 100 | Trace | NR |
| 11 | DMAP | CH3CO2Et | 81 | 78 | 88:12 |
| 12 | DMAP | CH3CN | 81 | 86d | 94:6 |
| 13 | DMAP | CH3CN | 25 | 36 | 46:54 |
| 14 | DMAP | CH3CN | 60 | 68 | 79:21 |
| 15 | DMAP | CH3CN | 81 | 50e | 90:10 |
 | |||||
| Entry | Base | Solvent | Temp./℃ | Yieldb/% | transc/cis |
| 1 | No base | CH3CN | 81 | NR | NR |
| 2 | NaOH | CH3CN | 81 | 41 | 45:55 |
| 3 | Cs2CO3 | CH3CN | 81 | 48 | 48:52 |
| 4 | Pyridine | CH3CN | 81 | 82c | 72:28 |
| 5 | TEBA | CH3CN | 81 | 56 | 80:20 |
| 6 | CTAB | CH3CN | 81 | 60 | 85:15 |
| 7 | DABCO | CH3CN | 81 | 70 | 72:28 |
| 8 | DMAP | CH3CN | 81 | 86 | 94:6 |
| 9 | DMAP | CH2Cl2 | 81 | 65 | 40:60 |
| 10 | DMAP | H2O | 100 | Trace | NR |
| 11 | DMAP | CH3CO2Et | 81 | 78 | 88:12 |
| 12 | DMAP | CH3CN | 81 | 86d | 94:6 |
| 13 | DMAP | CH3CN | 25 | 36 | 46:54 |
| 14 | DMAP | CH3CN | 60 | 68 | 79:21 |
| 15 | DMAP | CH3CN | 81 | 50e | 90:10 |
 | ||||||
| Entry | R1 | R2 | R3 | 4 | Yieldb/% | trans/cisc |
| 1 | Ph | 4-ClC6H4 | Me | 4a | 62 | 6:1 |
| 2 | Ph | 4-BrC6H4 | Me | 4b | 67 | 8:1 |
| 3 | Ph | 2-O2NC6H4 | Me | 4c | 84 | 11:1 |
| 4 | Ph | 2-ClC6H4 | Me | 4d | 85 | 12:1 |
| 5 | Ph | 2, 4-Cl2C6H4 | Me | 4e | 79 | 10:1 |
| 6 | 4-CH3C6H4 | 4-ClC6H4 | Me | 4f | 62 | 15:1 |
| 7 | 4-CH3C6H4 | 4-BrC6H4 | Me | 4g | 65 | 11:1 |
| 8 | 4-CH3C6H4 | 2-O2NC6H4 | Me | 4h | 86 | 18:1 |
| 9 | 4-CH3C6H4 | 2-ClC6H4 | Me | 4i | 86 | 17:1 |
| 10 | 4-CH3C6H4 | 2, 4-Cl2C6H4 | Me | 4j | 80 | 15:1 |
| 11 | 4-CH3C6H4 | Ph | Me | 4k | 65 | 6:1 |
| 12 | 4-ClC6H4 | 4-ClC6H4 | Me | 4l | 62 | 9:1 |
| 13 | 4-ClC6H4 | 4-BrC6H4 | Me | 4m | 66 | 8:1 |
| 14 | 4-ClC6H4 | 2-O2NC6H4 | Me | 4n | 58 | > 25:1 |
| 15 | 4-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4o | 82 | 15:1 |
| 16 | 2-ClC6H4 | 4-ClC6H4 | Me | 4p | 77 | 11:1 |
| 17 | 2-ClC6H4 | 4-BrC6H4 | Me | 4q | 78 | 12:1 |
| 18 | 2-ClC6H4 | 2-O2NC6H4 | Me | 4r | 83 | 18:1 |
| 19 | 2-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4s | 80 | 13:1 |
| 20 | Ph | 2, 4-Cl2C6H4 | C(Me)3 | 4t | 89 | > 25:1 |
| 21 | 4-CH3C6H4 | 2-O2NC6H4 | C(Me)3 | 4u | 87 | > 25:1 |
| 22 | 2-ClC6H4 | 2-O2NC6H4 | C(Me)3 | 4v | 87 | > 25:1 |
| 23 | 4-ClC6H4 | 2, 4-Cl2C6H4 | C(Me)3 | 4w | 85 | > 25:1 |
 | ||||||
| Entry | R1 | R2 | R3 | 4 | Yieldb/% | trans/cisc |
| 1 | Ph | 4-ClC6H4 | Me | 4a | 62 | 6:1 |
| 2 | Ph | 4-BrC6H4 | Me | 4b | 67 | 8:1 |
| 3 | Ph | 2-O2NC6H4 | Me | 4c | 84 | 11:1 |
| 4 | Ph | 2-ClC6H4 | Me | 4d | 85 | 12:1 |
| 5 | Ph | 2, 4-Cl2C6H4 | Me | 4e | 79 | 10:1 |
| 6 | 4-CH3C6H4 | 4-ClC6H4 | Me | 4f | 62 | 15:1 |
| 7 | 4-CH3C6H4 | 4-BrC6H4 | Me | 4g | 65 | 11:1 |
| 8 | 4-CH3C6H4 | 2-O2NC6H4 | Me | 4h | 86 | 18:1 |
| 9 | 4-CH3C6H4 | 2-ClC6H4 | Me | 4i | 86 | 17:1 |
| 10 | 4-CH3C6H4 | 2, 4-Cl2C6H4 | Me | 4j | 80 | 15:1 |
| 11 | 4-CH3C6H4 | Ph | Me | 4k | 65 | 6:1 |
| 12 | 4-ClC6H4 | 4-ClC6H4 | Me | 4l | 62 | 9:1 |
| 13 | 4-ClC6H4 | 4-BrC6H4 | Me | 4m | 66 | 8:1 |
| 14 | 4-ClC6H4 | 2-O2NC6H4 | Me | 4n | 58 | > 25:1 |
| 15 | 4-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4o | 82 | 15:1 |
| 16 | 2-ClC6H4 | 4-ClC6H4 | Me | 4p | 77 | 11:1 |
| 17 | 2-ClC6H4 | 4-BrC6H4 | Me | 4q | 78 | 12:1 |
| 18 | 2-ClC6H4 | 2-O2NC6H4 | Me | 4r | 83 | 18:1 |
| 19 | 2-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4s | 80 | 13:1 |
| 20 | Ph | 2, 4-Cl2C6H4 | C(Me)3 | 4t | 89 | > 25:1 |
| 21 | 4-CH3C6H4 | 2-O2NC6H4 | C(Me)3 | 4u | 87 | > 25:1 |
| 22 | 2-ClC6H4 | 2-O2NC6H4 | C(Me)3 | 4v | 87 | > 25:1 |
| 23 | 4-ClC6H4 | 2, 4-Cl2C6H4 | C(Me)3 | 4w | 85 | > 25:1 |
| [1] |
(a) Kinder, F. R. J.; Wang, R.-M.; Bauta, W. E.; Bair, K. W. M. Bioorg. Med. Chem. Lett. 1996, 6, 1029.
doi: 10.1016/0960-894X(96)00167-9 |
|
(b) Wessjohann, L. A.; Brandt, W. Chem. Rev. 2003, 103, 1625.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(c) Chen, D. Y.-K.; Pouwer, R. H.; Richard, J.-A. Chem. Soc. Rev. 2012, 41, 4631.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(d) Djerassi, C.; Doss, G. A. New J. Chem. 1990, 14, 713.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(e) Donaldson, W. A. Tetrahedron 2001, 57, 8589.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(f) Faust, R. Angew. Chem. 2001, 113, 2312.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(g) Qian, P.; Du, B. G.; Song, R. C.; Wu, X. D.; Mei, H. B.; Han, J. L.; Pan, Y. J. Org. Chem. 2016, 81, 6546.
doi: 10.1016/0960-894X(96)00167-9 |
|
| [2] |
Sampson P. B. Liu Y. Patel N. K. Feher M. Forrest B. J. Med. Chem. 2015 58 130.
doi: 10.1021/jm500537u |
| [3] |
Sahlberg C. Engelhardt P. J. Med. Chem. 1999 42 4150.
doi: 10.1021/jm990095j |
| [4] |
McMorris T. C. Kelner M. J. Wang W. Yu J. Estes L. A. Taetle R. J. Nat. Prod. 1996 59 896.
doi: 10.1021/np960450y |
| [5] |
(a) Cordero, F. M.; Pisaneschi, F.; Salvati, M.; Paschetta, V.; Ollivier, J.; Salaun, J.; Brandi, A. J. Org. Chem. 2003, 68, 3271.
doi: 10.1021/jo034003g |
|
(b) Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811.
doi: 10.1021/jo034003g |
|
| [6] |
(a) Lebel, H.; Marcoux, J. F.; Molinaro, C.; Charette, A. B. Chem. Rev. 2003, 103, 977.
doi: 10.1021/cr010007e |
|
(b) Kulinkovich, O. G.; Meijere, D. A. Chem. Rev. 2000, 100, 2789.
doi: 10.1021/cr010007e |
|
|
(c) Mukherjee, P.; Das, A. R. J. Org. Chem. 2017, 82, 2794.
doi: 10.1021/cr010007e |
|
| [7] |
Papageorgiou C. D. Cubillo de Dios M. A. Ley S. V. Gaunt M. J. Angew. Chem. Int. Ed. 2004 43 4641.
doi: 10.1002/anie.200460234 |
| [8] |
(a) Sun, X. L.; Tang, Y. Acc. Chem. Res. 2008, 41, 937.
doi: 10.1021/ar800108z |
|
(b) Kakei, H.; Sone, T.; Sohtome, Y.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2007, 129, 13410.
doi: 10.1021/ar800108z |
|
|
(c) Wang, J.; Liu, X. H.; Dong, S. X.; Lin, L. L.; Feng, X. M. J. Org. Chem. 2013, 78, 6322.
doi: 10.1021/ar800108z |
|
|
(d) Guo, J.; Liu, Y. B.; Li, X. Q.; Liu, X. H.; Lin, L. L.; Feng, X. M. Chem. Sci. 2016, 7, 2717.
doi: 10.1021/ar800108z |
|
| [9] |
(a) Sawada, T.; Nakada, M. Org. Lett. 2013, 15, 1004.
doi: 10.1021/ol303459x |
|
(b) Lindsay; V. N. G.; Nicolas, C.; Charette, A. B. J. Am. Chem. Soc. 2011, 133, 8972.
doi: 10.1021/ol303459x |
|
|
(c) Xu, X.; Zhu, S.; Cui, X.; Wojtas, L.; Zhang, X. P. Angew. Chem. 2013, 125, 12073.
doi: 10.1021/ol303459x |
|
|
(d) Xu, Z.-H.; Zhu, S.-N.; Sun, X.-L.; Tang, Y.; Dai, L.-X. Chem. Commun. 2007, 38, 1960.
doi: 10.1021/ol303459x |
|
| [10] |
(a) Arai, S.; Nakayama, K.; Hatano, K.; Shioiri, T. J. Org. Chem. 1998, 63, 9572.
doi: 10.1021/jo981409y |
|
(b) Miyagawa, T.; Tatenuma, T.; Tadokoro, M.; Satoh, T. Tetrahedron, 2008, 64, 5279.
doi: 10.1021/jo981409y |
|
| [11] |
(a) Newcomb, E. T.; Ferreira, E. M. Org. Lett. 2013, 15, 1772.
doi: 10.1021/ol400625f |
|
(b) Robinson, A.; Aggarwal, V. K. Angew. Chem. 2010, 122, 6823.
doi: 10.1021/ol400625f |
|
| [12] | Yuan Z.-B. Fang X.-X. Li X.-Y. Wu J. Yao H.-Q. Lin A.-J. J. Org. Chem. 2015 80 1112. |
| [13] |
Pyne S. G. Dong Z. Skelton B. W. White A. H. J. Org. Chem. 1997 62 2337.
doi: 10.1021/jo962216i |
| [14] |
Hanessian S. Andreotti D. Gomtsyan A. J. Am. Chem. Soc. 1995 117 10393.
doi: 10.1021/ja00146a029 |
| [15] |
Kimber M. C. Taylor D. K. J. Org. Chem. 2002 67 3142.
doi: 10.1021/jo0110496 |
| [16] | Avery T. D. Jenkins N. F. Kimber M. C. Lupton D. W. Taylor D. K. Chem. Commun. 2002 33 28. |
| [17] |
Wang Q. Song X. K. Chen J. Yan C. G. J. Comb. Chem. 2009 11 1007.
doi: 10.1021/cc900005v |
| [18] | Ošeka M. Noole A. Žari S. Öeren M. Järving I. Lopp M. Kanger T. Eur. J. Org. Chem. 2014 17 3599. |
| [19] |
Ren Z. J. Cao W. G. Tong W. Q. Chen J. Deng H. M. Wu D. Y. Synth. Commun. 2008 38 2200.
doi: 10.1080/00397910802029406 |
| [20] |
Ren Z. J. Cao W. G. Chen J. Chen Y. L. Deng H. M. Shao M. Wu D. Y. Tetrahedron 2008 64 5156.
doi: 10.1016/j.tet.2008.03.049 |
| [21] |
Li J. H. Feng T. F. Du D. M. J. Org. Chem. 2015 80 11369.
doi: 10.1021/acs.joc.5b01940 |
| [22] |
(a) Ablajan, K.; Zeynepgul, E.; Wang, L. J.; Feng, J. Tetrahedron 2014, 70, 3976.
doi: 10.1016/j.tet.2014.04.088 |
|
(b) Wang, L. J.; Ablajan, K.; Feng, J. Ultrason. Sonochem. 2015, 22, 113.
doi: 10.1016/j.tet.2014.04.088 |
|
|
(c) Li, W. B.; Reyhangul, R.; Ablajan, K.; Zulpiya, G. Tetrahedron 2017, 73, 164.
doi: 10.1016/j.tet.2014.04.088 |
|
| [23] |
(a) Khan, A.; Lal, M.; Sidick Basha, R. Synthesis 2013, 45, 406.
doi: 10.1055/s-00000084 |
|
(b) Wang, Q.-F.; Hou, H.; Hui, L.; Yan, C.-G. J. Org. Chem. 2009, 74, 7403.
doi: 10.1055/s-00000084 |
|
|
(c) Chuang, C.-P.; Chen, K.-P. Tetrahedron 2012, 68, 1401.
doi: 10.1055/s-00000084 |
| [1] | 张文生, 李焱, 崔海燕, 苏小莉, 徐素鹏. 邻甲酰基苯甲酸甲酯还原胺化/内酰胺化一锅法合成N-取代异吲哚-1-酮[J]. 有机化学, 2022, 42(8): 2456-2461. |
| [2] | 张玉荣, 王晗, 茆勇军, 施世良. 镍催化丁二烯、亚胺和烯基硼酸的三组分偶联反应[J]. 有机化学, 2022, 42(4): 1198-1209. |
| [3] | 罗享豪, 谢益碧, 黄年玉, 王龙. 基于原位捕获异腈的Ugi四组分反应及其后修饰串联反应: 一锅法合成含氮杂环化合物[J]. 有机化学, 2022, 42(3): 838-846. |
| [4] | 穆思宇, 李红霞, 伍智林, 彭俊梅, 陈锦杨, 何卫民. 电催化肼、丙二酮和2-溴丙二酸二乙酯三组分合成4-溴吡唑[J]. 有机化学, 2022, 42(12): 4292-4299. |
| [5] | 马蔚青, 韩莹, 孙晶, 颜朝国. 三组分反应高效合成螺[环戊烷-1,3'-吲哚啉]衍生物[J]. 有机化学, 2021, 41(8): 3180-3191. |
| [6] | 刘金妮, 谢益碧, 阳青青, 黄年玉, 王龙. 基于原位捕获胺的Ugi四组分反应及其后修饰串联环化反应:“一锅法”合成六元、七元杂环化合物[J]. 有机化学, 2021, 41(6): 2374-2383. |
| [7] | 李雨青, 施世良. 镍催化的丁二烯、醛、炔和氢氯二茂锆的多组分偶联反应合成1,4-二烯[J]. 有机化学, 2021, 41(5): 1939-1948. |
| [8] | 殷国栋, 李源, 范玲. 四氯化锆催化合成嘧啶并[4,5-b]喹啉-2,4(1H,3H)-二酮和11H-茚[1,2-b]喹啉-11-酮[J]. 有机化学, 2021, 41(3): 1234-1240. |
| [9] | 王琦, 朱柏燃, 杨光, 马献涛, 徐清. 无碱条件下直接多组分反应选择性合成非对称含氮杂芳基硫醚[J]. 有机化学, 2021, 41(3): 1193-1199. |
| [10] | 闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖. 苯炔参与的去芳构化反应研究进展[J]. 有机化学, 2021, 41(2): 455-470. |
| [11] | 秦锋, 汤琳, 黄飞, 李晓悦, 张武. 铜催化氧化和Aza-Diels-Alder反应三组分合成喹啉[J]. 有机化学, 2021, 41(1): 318-324. |
| [12] | 肖立伟, 刘光仙, 李政, 任萍, 任丽磊, 孔洁. 低共熔溶剂促进N-取代十氢吖啶-1,8-二酮类化合物的合成[J]. 有机化学, 2020, 40(9): 2988-2993. |
| [13] | 孙晓华, 孙传策, 冯立军, 康从民. 串联环化反应合成噻吩并[2,3-d]嘧啶类化合物的研究进展[J]. 有机化学, 2020, 40(9): 2626-2640. |
| [14] | 周聪, 李渺, 于金涛, 孙松, 成江. 以二氧化碳为C1合成子的羧基化/环化反应研究进展[J]. 有机化学, 2020, 40(8): 2221-2231. |
| [15] | 何淑旺, 颜世强, 郭伟, 翟光喜, 张伟. 苯乙烯一锅法合成氨基醇[J]. 有机化学, 2020, 40(7): 2094-2098. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||